Postnatal Development and Distribution of Sympathetic Innervation in Mouse Skeletal Muscle
- PMID: 29966393
- PMCID: PMC6073285
- DOI: 10.3390/ijms19071935
Postnatal Development and Distribution of Sympathetic Innervation in Mouse Skeletal Muscle
Abstract
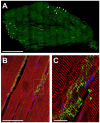
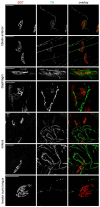
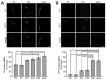
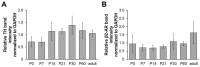
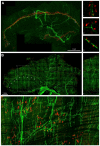
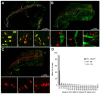
Vertebrate neuromuscular junctions (NMJs) have been conceived as tripartite synapses composed of motor neuron, Schwann cell, and muscle fiber. Recent work has shown the presence of sympathetic neurons in the immediate vicinity of NMJs and experimental and clinical findings suggest that this plays an eminent role in adult NMJ biology. The present study examined the postnatal development and distribution of sympathetic innervation in different muscles using immunofluorescence, confocal microscopy, and Western blot. This demonstrates the proximity of sympathetic neurons in diaphragm, extensor digitorum longus, tibialis anterior, soleus, and levator auris longus muscles. In extensor digitorum longus muscle, sympathetic innervation of NMJs was quantified from perinatal to adult stage and found to increase up to two months of age. In diaphragm muscle, an extensive network of sympathetic neurons was prominent along the characteristic central synapse band. In summary, these data demonstrate that an elaborate sympathetic innervation is present in several mouse skeletal muscles and that this is often next to NMJs. Although the presence of sympathetic neurons at the perisynaptic region of NMJs increased during postnatal development, many synapses were already close to sympathetic neurons at birth. Potential implications of these findings for treatment of neuromuscular diseases are discussed.
Keywords: endplate; neuromuscular junction; neuropeptide Y; sympathetic neuron; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Subcellular localization of the glutamate transporters GLAST and GLT at the neuromuscular junction in rodents.Neuroscience. 2007 Mar 16;145(2):579-91. doi: 10.1016/j.neuroscience.2006.12.041. Epub 2007 Feb 6. Neuroscience. 2007. PMID: 17289278
-
Shared resistance to aging and ALS in neuromuscular junctions of specific muscles.PLoS One. 2012;7(4):e34640. doi: 10.1371/journal.pone.0034640. Epub 2012 Apr 2. PLoS One. 2012. PMID: 22485182 Free PMC article.
-
Prevalence and elimination of sibling neurite convergence in motor units supplying neonatal and adult mouse skeletal muscle.J Comp Neurol. 2012 Oct 1;520(14):3203-16. doi: 10.1002/cne.23091. J Comp Neurol. 2012. PMID: 22430826
-
Sympathetic innervation in skeletal muscle and its role at the neuromuscular junction.J Muscle Res Cell Motil. 2024 Jun;45(2):79-86. doi: 10.1007/s10974-024-09665-9. Epub 2024 Feb 17. J Muscle Res Cell Motil. 2024. PMID: 38367152 Free PMC article. Review.
-
Pre- and post-synaptic roles of action potential activity in synapse elimination revealed by using ectopic neuromuscular junction formation by a foreign nerve.Neurosci Lett. 2020 Mar 23;722:134835. doi: 10.1016/j.neulet.2020.134835. Epub 2020 Feb 10. Neurosci Lett. 2020. PMID: 32057925 Review.
Cited by
-
The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability.Acta Physiol (Oxf). 2019 Mar;225(3):e13195. doi: 10.1111/apha.13195. Epub 2018 Oct 22. Acta Physiol (Oxf). 2019. PMID: 30269419 Free PMC article.
-
A Perspective on Electrical Stimulation and Sympathetic Regeneration in Peripheral Nerve Injuries.Neurotrauma Rep. 2024 Mar 4;5(1):172-180. doi: 10.1089/neur.2023.0133. eCollection 2024. Neurotrauma Rep. 2024. PMID: 38463421 Free PMC article.
-
Alx3 deficiency disrupts energy homeostasis, alters body composition, and impairs hypothalamic regulation of food intake.Cell Mol Life Sci. 2024 Aug 12;81(1):343. doi: 10.1007/s00018-024-05384-z. Cell Mol Life Sci. 2024. PMID: 39129011 Free PMC article.
-
State of Knowledge on Molecular Adaptations to Exercise in Humans: Historical Perspectives and Future Directions.Compr Physiol. 2022 Mar 9;12(2):3193-3279. doi: 10.1002/cphy.c200033. Compr Physiol. 2022. PMID: 35578962 Free PMC article.
-
Aging Blunts Sympathetic Neuron Regulation of Motoneurons Synaptic Vesicle Release Mediated by β1- and α2B-Adrenergic Receptors in Geriatric Mice.J Gerontol A Biol Sci Med Sci. 2020 Jul 13;75(8):1473-1480. doi: 10.1093/gerona/glaa022. J Gerontol A Biol Sci Med Sci. 2020. PMID: 31956900 Free PMC article.
References
-
- Khan M.M., Lustrino D., Silveira W.A., Wild F., Straka T., Issop Y., O’Connor E., Cox D., Reischl M., Marquardt T., et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. 2016;113:746–750. doi: 10.1073/pnas.1524272113. - DOI - PMC - PubMed
-
- Boeke J. Die motorische Endplatte bei den höheren Vertebraten, ihre Entwickelung, Form und Zusammenhang mit der Muskelfaser. Anat Anz. 1909;35:240–256.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

