Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy
- PMID: 29955439
- PMCID: PMC6015743
- DOI: 10.1038/s41551-017-0187-5
Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy
Abstract
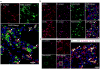
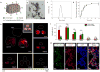
Bacterial resistance to antibiotics has made it necessary to resort to antibiotics that have considerable toxicities. Here, we show that the cyclic 9-amino acid peptide CARGGLKSC (CARG), identified via phage display on Staphylococcus aureus (S. aureus) bacteria and through in vivo screening in mice with S. aureus-induced lung infections, increases the antibacterial activity of CARG-conjugated vancomycin-loaded nanoparticles in S. aureus-infected tissues and reduces the needed overall systemic dose, minimizing side effects. CARG binds specifically to S. aureus bacteria but not Pseudomonas bacteria in vitro, selectively accumulates in S. aureus-infected lungs and skin of mice but not in non-infected tissue and Pseudomonas-infected tissue, and significantly enhances the accumulation of intravenously injected vancomycin-loaded porous silicon nanoparticles bearing the peptide in S. aureus-infected mouse lung tissue. The targeted nanoparticles more effectively suppress staphylococcal infections in vivo relative to equivalent doses of untargeted vancomycin nanoparticles or of free vancomycin. The therapeutic delivery of antibiotic-carrying nanoparticles bearing peptides targeting infected tissue may help combat difficult-to-treat infections.
Conflict of interest statement
Competing financial interests M.J.S is a scientific founder of Spinnaker Biosciences, Inc., and has an equity interest in the company. Although the R01 AI132413-01 grant has been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Spinnaker Biosciences, Inc., the research findings included in this particular publication may not necessarily relate to the interests of Spinnaker Biosciences, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. All the other authors declare no competing financial interests.
Figures



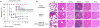
Comment in
-
Infection-targeted bactericidal nanoparticles.Nat Biomed Eng. 2018 Feb;2(2):56-57. doi: 10.1038/s41551-018-0199-9. Nat Biomed Eng. 2018. PMID: 31015624 No abstract available.
Similar articles
-
99mTc-Labeled UBI 29-41 peptide for monitoring the efficacy of antibacterial agents in mice infected with Staphylococcus aureus.J Nucl Med. 2004 Feb;45(2):321-6. J Nucl Med. 2004. PMID: 14960656
-
Skin and muscle permeating antibacterial nanoparticles for treating Staphylococcus aureus infected wounds.J Biomed Mater Res B Appl Biomater. 2016 May;104(4):797-807. doi: 10.1002/jbm.b.33635. Epub 2016 Feb 21. J Biomed Mater Res B Appl Biomater. 2016. PMID: 26898355
-
Potent antibacterial nanoparticles for pathogenic bacteria.ACS Appl Mater Interfaces. 2015 Jan 28;7(3):2046-54. doi: 10.1021/am507919m. Epub 2015 Jan 13. ACS Appl Mater Interfaces. 2015. PMID: 25584802
-
A review on nanosystems as an effective approach against infections of Staphylococcus aureus.Int J Nanomedicine. 2018 Nov 9;13:7333-7347. doi: 10.2147/IJN.S169935. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30519018 Free PMC article. Review.
-
Skin and soft tissue infection.Indian J Pediatr. 2001 Jul;68 Suppl 3:S46-50. Indian J Pediatr. 2001. PMID: 11980459 Review.
Cited by
-
Periodicity Pitch Perception.Front Neurosci. 2020 Jun 4;14:486. doi: 10.3389/fnins.2020.00486. eCollection 2020. Front Neurosci. 2020. PMID: 32581672 Free PMC article.
-
Tunneling Nanotubes: A New Target for Nanomedicine?Int J Mol Sci. 2022 Feb 17;23(4):2237. doi: 10.3390/ijms23042237. Int J Mol Sci. 2022. PMID: 35216348 Free PMC article. Review.
-
Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery.Adv Mater. 2019 Dec;31(49):e1903637. doi: 10.1002/adma.201903637. Epub 2019 Sep 30. Adv Mater. 2019. PMID: 31566258 Free PMC article. Review.
-
Retracted Article: Organometallic Ag nanostructures prepared using Hypericum perforatum extract are highly effective against multidrug-resistant bacteria.RSC Adv. 2018 Aug 29;8(53):30562-30572. doi: 10.1039/c8ra05655b. eCollection 2018 Aug 24. RSC Adv. 2018. Retraction in: RSC Adv. 2019 Apr 12;9(20):11459. doi: 10.1039/c9ra90026h. PMID: 35546844 Free PMC article. Retracted.
-
Co-Delivery of Nano-Silver and Vancomycin via Silica Nanopollens for Enhanced Antibacterial Functions.Antibiotics (Basel). 2022 May 18;11(5):685. doi: 10.3390/antibiotics11050685. Antibiotics (Basel). 2022. PMID: 35625329 Free PMC article.
References
-
- Moran GJ, et al. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. New England Journal of Medicine. 2006;355:666–674. - PubMed
-
- Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. - PubMed
-
- Moellering RC. MRSA: the first half century. Journal of Antimicrobial Chemotherapy. 2012;67:4–11. - PubMed
-
- de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43:417–440. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

