Automated Chemical Oligosaccharide Synthesis: Novel Approach to Traditional Challenges
- PMID: 29953217
- PMCID: PMC6522228
- DOI: 10.1021/acs.chemrev.8b00051
Automated Chemical Oligosaccharide Synthesis: Novel Approach to Traditional Challenges
Abstract
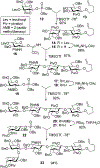
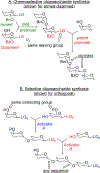
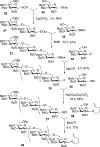
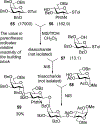
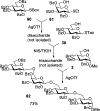
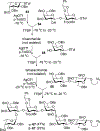
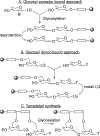
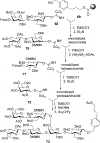
Advances in carbohydrate chemistry have certainly made common oligosaccharides much more accessible. However, many current methods still rely heavily upon specialized knowledge of carbohydrate chemistry. The application of automated technologies to chemical and life science applications such as genomics and proteomics represents a vibrant field. These automated technologies also present opportunities for their application to organic synthesis, including that of the synthesis of oligosaccharides. However, application of automated methods to the synthesis of carbohydrates is an underdeveloped area as compared to other classes of biomolecules. The overarching goal of this review article is to present the advances that have been made at the interface of carbohydrate chemistry and automated technology.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures







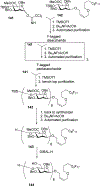
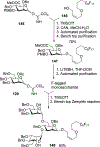
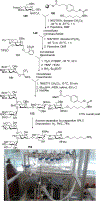
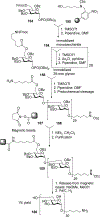




Similar articles
-
Toward Automated Enzymatic Synthesis of Oligosaccharides.Chem Rev. 2018 Sep 12;118(17):8151-8187. doi: 10.1021/acs.chemrev.8b00066. Epub 2018 Jul 16. Chem Rev. 2018. PMID: 30011195 Review.
-
Catalytic Glycosylations in Oligosaccharide Synthesis.Chem Rev. 2018 Sep 12;118(17):8285-8358. doi: 10.1021/acs.chemrev.8b00144. Epub 2018 Jul 3. Chem Rev. 2018. PMID: 29969248 Review.
-
Automated carbohydrate synthesis to drive chemical glycomics.Chem Commun (Camb). 2003 May 21;(10):1115-21. doi: 10.1039/b210230g. Chem Commun (Camb). 2003. PMID: 12778696
-
Programmable One-Pot Synthesis of Oligosaccharides.Biochemistry. 2020 Sep 1;59(34):3078-3088. doi: 10.1021/acs.biochem.9b00613. Epub 2019 Sep 6. Biochemistry. 2020. PMID: 31454239 Review.
-
Automated solid-phase synthesis of oligosaccharides.Science. 2001 Feb 23;291(5508):1523-7. doi: 10.1126/science.1057324. Epub 2001 Feb 1. Science. 2001. PMID: 11222853
Cited by
-
Site-Selective and Stereoselective O-Alkylation of Glycosides by Rh(II)-Catalyzed Carbenoid Insertion.J Am Chem Soc. 2019 Dec 18;141(50):19902-19910. doi: 10.1021/jacs.9b11262. Epub 2019 Dec 3. J Am Chem Soc. 2019. PMID: 31739665 Free PMC article.
-
Bromine-Promoted Glycosidation of Conformationally Superarmed Thioglycosides.Chemistry. 2019 Sep 12;25(51):11831-11836. doi: 10.1002/chem.201901969. Epub 2019 Aug 20. Chemistry. 2019. PMID: 31286579 Free PMC article.
-
Manual and Automated Syntheses of the N-Linked Glycoprotein Core Glycans.J Org Chem. 2019 Jun 7;84(11):6576-6588. doi: 10.1021/acs.joc.8b03056. Epub 2019 May 17. J Org Chem. 2019. PMID: 31066275 Free PMC article.
-
Preparative scale purification of natural glycans by closed-loop recycle HPLC.Anal Biochem. 2020 Jun 15;599:113702. doi: 10.1016/j.ab.2020.113702. Epub 2020 Apr 8. Anal Biochem. 2020. PMID: 32277906 Free PMC article.
-
Fluoride Migration Catalysis Enables Simple, Stereoselective, and Iterative Glycosylation.J Am Chem Soc. 2020 Apr 15;142(15):7235-7242. doi: 10.1021/jacs.0c03165. Epub 2020 Apr 1. J Am Chem Soc. 2020. PMID: 32207615 Free PMC article.
References
-
- Stick RV; Williams SJ Carbohydrates: The Essential Molecules of Life, 2nd ed.; Elsevier: Amsterdam, 2009.
-
- Benoff S Carbohydrates and fertilization: an overview. Mol. Hum. Reprod 1997, 3, 599–637. - PubMed
-
- Varki A; Cummings RD; Esko JD; Freeze HH; Bertozzi CR; Stanley P; Hart GW; Etzler ME Essentials of glycobiology, 2nd ed.; CSH Laboratory Press: New York, 2009. - PubMed
-
- Cipolla L; Araújo AC; Bini D; Gabrielli L; Russo L; Shaikh N Discovery and design of carbohydrate-based therapeutics. Expert Opin. Drug Discovery 2010, 5, 721–737. - PubMed
-
- Witczak ZJ Carbohydrates as new and old targets for future drug design In Carbohydrates in Drug Design; Witczak ZJ, Nieforth KA, Eds.; Marcel Dekker, Inc.: New York, 1997; pp 1–37.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

