HIV-1 Subtype C-Infected Children with Exceptional Neutralization Breadth Exhibit Polyclonal Responses Targeting Known Epitopes
- PMID: 29950423
- PMCID: PMC6096808
- DOI: 10.1128/JVI.00878-18
HIV-1 Subtype C-Infected Children with Exceptional Neutralization Breadth Exhibit Polyclonal Responses Targeting Known Epitopes
Abstract
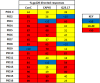
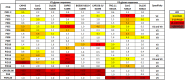
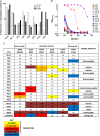
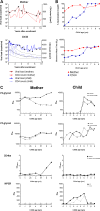
We have previously shown that HIV-1-infected children develop broader and more potent neutralizing antibody responses than adults. This study aimed to determine the antibody specificities in 16 HIV-1 subtype C-infected children who displayed exceptional neutralization breadth on a 22-multisubtype virus panel. All children were antiretroviral treatment (ART) naive with normal CD4 counts despite being infected for a median of 10.1 years with high viral loads. The specificity of broadly neutralizing antibodies (bNAbs) was determined using epitope-ablating mutants, chimeric constructs, and depletion or inhibition of activity with peptides and glycoproteins. We found that bNAbs in children largely targeted previously defined epitopes, including the V2-glycan, V3-glycan, CD4bs, and gp120-gp41 interface. Remarkably, 63% of children had antibodies targeting 2 or 3 and, in one case, 4 of these bNAb epitopes. Longitudinal analysis of plasma from a mother-child pair over 9 years showed that while they both had similar neutralization profiles, the antibody specificities differed. The mother developed antibodies targeting the V2-glycan and CD4bs, whereas bNAb specificities in the child could not be mapped until 6 years, when a minor V2-glycan response appeared. The child also developed high-titer membrane-proximal external region (MPER) binding antibodies not seen in the mother, although these were not a major bNAb specificity. Overall, exceptional neutralization breadth in this group of children may be the result of extended exposure to high antigenic load in the context of an intact immune system, which allowed for the activation of multiple B cell lineages and the generation of polyclonal responses targeting several bNAb epitopes.IMPORTANCE An HIV vaccine is likely to require bNAbs, which have been shown to prevent HIV acquisition in nonhuman primates. Recent evidence suggests that HIV-infected children are inherently better at generating bNAbs than adults. Here, we show that exceptional neutralization breadth in a group of viremic HIV-1 subtype C-infected children was due to the presence of polyclonal bNAb responses. These bNAbs targeted multiple epitopes on the HIV envelope glycoprotein previously defined in adult infection, suggesting that the immature immune system recognizes HIV antigens similarly. Since elicitation of a polyclonal bNAb response is the basis of next-generation HIV envelope vaccines, further studies of how bNAb lineages are stimulated in children is warranted. Furthermore, our findings suggest that children may respond particularly well to vaccines designed to elicit antibodies to multiple bNAb epitopes.
Keywords: HIV envelope targets; HIV vaccines; HIV-1 subtype C; bNAbs; epitope mapping; mother-child transmission; neutralization breadth; neutralizing antibodies; pediatric immunology.
Copyright © 2018 Ditse et al.
Figures




Similar articles
-
Positive Selection at Key Residues in the HIV Envelope Distinguishes Broad and Strain-Specific Plasma Neutralizing Antibodies.J Virol. 2019 Mar 5;93(6):e01685-18. doi: 10.1128/JVI.01685-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30567996 Free PMC article.
-
Viral Characteristics Associated with Maintenance of Elite Neutralizing Activity in Chronically HIV-1 Clade C-Infected Monozygotic Pediatric Twins.J Virol. 2019 Aug 13;93(17):e00654-19. doi: 10.1128/JVI.00654-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31217240 Free PMC article.
-
Anti-V3/Glycan and Anti-MPER Neutralizing Antibodies, but Not Anti-V2/Glycan Site Antibodies, Are Strongly Associated with Greater Anti-HIV-1 Neutralization Breadth and Potency.J Virol. 2015 May;89(10):5264-75. doi: 10.1128/JVI.00129-15. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673728 Free PMC article.
-
Neutralizing antibodies for HIV-1 prevention.Curr Opin HIV AIDS. 2019 Jul;14(4):318-324. doi: 10.1097/COH.0000000000000556. Curr Opin HIV AIDS. 2019. PMID: 31082819 Free PMC article. Review.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
Cited by
-
Impact of HIV-1 Diversity on Its Sensitivity to Neutralization.Vaccines (Basel). 2019 Jul 25;7(3):74. doi: 10.3390/vaccines7030074. Vaccines (Basel). 2019. PMID: 31349655 Free PMC article. Review.
-
Understanding Antibody Responses in Early Life: Baby Steps towards Developing an Effective Influenza Vaccine.Viruses. 2021 Jul 17;13(7):1392. doi: 10.3390/v13071392. Viruses. 2021. PMID: 34372597 Free PMC article. Review.
-
Dependence on a variable residue limits the breadth of an HIV MPER neutralizing antibody, despite convergent evolution with broadly neutralizing antibodies.PLoS Pathog. 2022 Sep 2;18(9):e1010450. doi: 10.1371/journal.ppat.1010450. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36054228 Free PMC article.
-
Design and immunogenicity of an HIV-1 clade C pediatric envelope glycoprotein stabilized by multiple platforms.bioRxiv [Preprint]. 2024 Sep 19:2024.09.14.613016. doi: 10.1101/2024.09.14.613016. bioRxiv. 2024. PMID: 39345501 Free PMC article. Preprint.
-
Frequent Development of Broadly Neutralizing Antibodies in Early Life in a Large Cohort of Children With Human Immunodeficiency Virus.J Infect Dis. 2022 May 16;225(10):1731-1740. doi: 10.1093/infdis/jiab629. J Infect Dis. 2022. PMID: 34962990 Free PMC article.
References
-
- Judd A, Ferrand RA, Jungmann E, Foster C, Masters J, Rice B, Lyall H, Tookey PA, Prime K. 2009. Vertically acquired HIV diagnosed in adolescence and early adulthood in the United Kingdom and Ireland: findings from national surveillance. HIV Med 10:253–256. doi:10.1111/j.1468-1293.2008.00676.x. - DOI - PubMed
-
- Muenchhoff M, Adland E, Karimanzira O, Crowther C, Pace M, Csala A, Leitman E, Moonsamy A, McGregor C, Hurst J, Groll A, Mori M, Sinmyee S, Thobakgale C, Tudor-Williams G, Prendergast AJ, Kloverpris H, Roider J, Leslie A, Shingadia D, Brits T, Daniels S, Frater J, Willberg CB, Walker BD, Ndung'u T, Jooste P, Moore PL, Morris L, Goulder P. 2016. Nonprogressing HIV-infected children share fundamental immunological features of nonpathogenic SIV infection. Sci Transl Med 8:358ra125. doi:10.1126/scitranslmed.aag1048. - DOI - PMC - PubMed
-
- Adland E, Paioni P, Thobakgale C, Laker L, Mori L, Muenchhoff M, Csala A, Clapson M, Flynn J, Novelli V, Hurst J, Naidoo V, Shapiro R, Huang KH, Frater J, Prendergast A, Prado JG, Ndung'u T, Walker BD, Carrington M, Jooste P, Goulder PJ. 2015. Discordant impact of HLA on viral replicative capacity and disease progression in pediatric and adult HIV infection. PLoS Pathog 11:e1004954. doi:10.1371/journal.ppat.1004954. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

