Attenuated Herpes Simplex Virus 1 (HSV-1) Expressing a Mutant Form of ICP6 Stimulates a Strong Immune Response That Protects Mice against HSV-1-Induced Corneal Disease
- PMID: 29950407
- PMCID: PMC6096795
- DOI: 10.1128/JVI.01036-18
Attenuated Herpes Simplex Virus 1 (HSV-1) Expressing a Mutant Form of ICP6 Stimulates a Strong Immune Response That Protects Mice against HSV-1-Induced Corneal Disease
Abstract
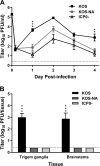
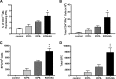
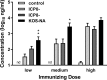
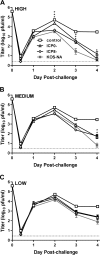
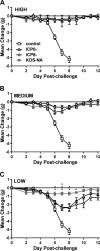
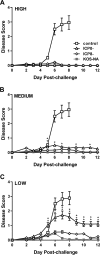
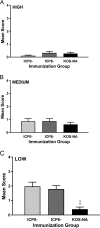
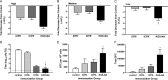
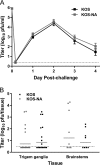
We previously isolated a herpes simplex virus 1 (HSV-1) mutant, KOS-NA, that carries two nonsynonymous mutations in UL39, resulting in L393P and R950H amino acid substitutions in infected cell protein 6 (ICP6). Our published data studying KOS-NA pathogenesis strongly suggest that one of these ICP6 substitutions expressed from KOS-NA, R950H, severely impaired acute viral replication in the eyes and trigeminal ganglia of mice after inoculation onto the cornea and consequently impaired establishment and reactivation from latency. Because of its significant neuroattenuation, we tested KOS-NA as a potential prophylactic vaccine against HSV-1 in a mouse model of corneal infection. KOS-NA stimulated stronger antibody and T cell responses than a replication-competent ICP0-null mutant and a replication-incompetent ICP8-null mutant optimized for immunogenicity. Immunizations with the ICP0-, ICP8-, and KOS-NA viruses all reduced replication of wild-type HSV-1 challenge virus in the corneal epithelium to similar extents. Low immunizing doses of KOS-NA and the ICP8- virus, but not the ICP0- virus, protected mice against eyelid disease (blepharitis). Notably, only KOS-NA protected almost completely against corneal disease (keratitis) and greatly reduced latent infection by challenge virus. Thus, vaccination of mice with KOS-NA prior to corneal challenge provides significant protection against HSV-1-mediated disease of the eye, even at a very low immunizing dose. These results suggest that KOS-NA may be the foundation of an effective prophylactic vaccine to prevent or limit HSV-1 ocular diseases.IMPORTANCE HSV-1 is a ubiquitous human pathogen that infects the majority of the world's population. Although most infections are asymptomatic, HSV-1 establishes lifelong latency in infected sensory neurons, from which it can reactivate to cause deadly encephalitis or potentially blinding eye disease. No clinically effective vaccine is available. In this study, we tested the protective potential of a neuroattenuated HSV-1 mutant (KOS-NA) as a vaccine in mice. We compared the effects of immunization with KOS-NA to those of two other attenuated viruses, a replication-competent (ICP0-) virus and a replication-incompetent (ICP8-) virus. Our data show that KOS-NA proved superior to the ICP0- and ICP8-null mutants in protecting mice from corneal disease and latent infection. With its significant neuroattenuation, severe impairment in establishing latency, and excellent protective effect, KOS-NA represents a significant discovery in the field of HSV-1 vaccine development.
Keywords: HSV-1; ICP6; cornea; herpes simplex virus; immunization; keratitis; mutant; ocular; vaccine.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication.Viruses. 2022 Apr 22;14(5):869. doi: 10.3390/v14050869. Viruses. 2022. PMID: 35632611 Free PMC article.
-
Herpes Simplex Virus 1 Mutant with Point Mutations in UL39 Is Impaired for Acute Viral Replication in Mice, Establishment of Latency, and Explant-Induced Reactivation.J Virol. 2018 Mar 14;92(7):e01654-17. doi: 10.1128/JVI.01654-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29321311 Free PMC article.
-
Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection.J Virol. 2002 Apr;76(8):3615-25. doi: 10.1128/jvi.76.8.3615-3625.2002. J Virol. 2002. PMID: 11907201 Free PMC article.
-
Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses.Viruses. 2021 Aug 18;13(8):1637. doi: 10.3390/v13081637. Viruses. 2021. PMID: 34452501 Free PMC article. Review.
-
Role of Herpes Simplex Virus Type 1 (HSV-1) Glycoprotein K (gK) Pathogenic CD8+ T Cells in Exacerbation of Eye Disease.Front Immunol. 2018 Dec 7;9:2895. doi: 10.3389/fimmu.2018.02895. eCollection 2018. Front Immunol. 2018. PMID: 30581441 Free PMC article. Review.
Cited by
-
Distinguishing Features of High- and Low-Dose Vaccine against Ocular HSV-1 Infection Correlates with Recognition of Specific HSV-1-Encoded Proteins.Immunohorizons. 2020 Oct 9;4(10):608-626. doi: 10.4049/immunohorizons.2000060. Immunohorizons. 2020. PMID: 33037098 Free PMC article.
-
A Single-Cycle Glycoprotein D Deletion Viral Vaccine Candidate, ΔgD-2, Elicits Polyfunctional Antibodies That Protect against Ocular Herpes Simplex Virus.J Virol. 2020 Jun 16;94(13):e00335-20. doi: 10.1128/JVI.00335-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295919 Free PMC article.
-
Herpes Simplex Virus 1 (HSV-1) 0ΔNLS Live-Attenuated Vaccine Protects against Ocular HSV-1 Infection in the Absence of Neutralizing Antibody in HSV-1 gB T Cell Receptor-Specific Transgenic Mice.J Virol. 2020 Nov 23;94(24):e01000-20. doi: 10.1128/JVI.01000-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999018 Free PMC article.
-
"Non-Essential" Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review.Viruses. 2020 Dec 23;13(1):17. doi: 10.3390/v13010017. Viruses. 2020. PMID: 33374862 Free PMC article. Review.
-
Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication.Viruses. 2022 Apr 22;14(5):869. doi: 10.3390/v14050869. Viruses. 2022. PMID: 35632611 Free PMC article.
References
-
- Roizman R, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601. In Knipe DM, Howley PM (ed), Fields virology, vol 2 Lippincott Williams & Wilkins, New York, NY.
-
- Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Wilhelmus KR, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting D, Asbell PA. 1994. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101:1871–1882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

