Actin dynamics in host-pathogen interaction
- PMID: 29935019
- PMCID: PMC6282728
- DOI: 10.1002/1873-3468.13173
Actin dynamics in host-pathogen interaction
Abstract
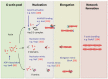
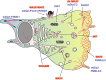
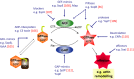
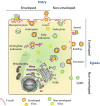
The actin cytoskeleton and Rho GTPase signaling to actin assembly are prime targets of bacterial and viral pathogens, simply because actin is involved in all motile and membrane remodeling processes, such as phagocytosis, macropinocytosis, endocytosis, exocytosis, vesicular trafficking and membrane fusion events, motility, and last but not least, autophagy. This article aims at providing an overview of the most prominent pathogen-induced or -hijacked actin structures, and an outlook on how future research might uncover additional, equally sophisticated interactions.
Keywords: actin dynamics; bacterial invasion; host-pathogen interaction; viral entry; virulence factors.
© 2018 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
Similar articles
-
The non-canonical roles of clathrin and actin in pathogen internalization, egress and spread.Nat Rev Microbiol. 2013 Aug;11(8):551-60. doi: 10.1038/nrmicro3072. Nat Rev Microbiol. 2013. PMID: 24020073 Review.
-
Manipulation of host membranes by bacterial effectors.Nat Rev Microbiol. 2011 Jul 18;9(9):635-46. doi: 10.1038/nrmicro2602. Nat Rev Microbiol. 2011. PMID: 21765451 Review.
-
Hijacking Host Cell Highways: Manipulation of the Host Actin Cytoskeleton by Obligate Intracellular Bacterial Pathogens.Front Cell Infect Microbiol. 2016 Sep 22;6:107. doi: 10.3389/fcimb.2016.00107. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27713866 Free PMC article. Review.
-
Pathogens and polymers: microbe-host interactions illuminate the cytoskeleton.J Cell Biol. 2011 Oct 3;195(1):7-17. doi: 10.1083/jcb.201103148. J Cell Biol. 2011. PMID: 21969466 Free PMC article. Review.
-
Caveolin-1-mediated Japanese encephalitis virus entry requires a two-step regulation of actin reorganization.Future Microbiol. 2016 Oct;11:1227-1248. doi: 10.2217/fmb-2016-0002. Epub 2016 Mar 17. Future Microbiol. 2016. PMID: 26986451
Cited by
-
Knockdown of CRAD suppresses the growth and promotes the apoptosis of human lung cancer cells via Claudin 4.Biosci Rep. 2020 Oct 30;40(10):BSR20201140. doi: 10.1042/BSR20201140. Biosci Rep. 2020. PMID: 33006362 Free PMC article.
-
Entry of Epidemic Keratoconjunctivitis-Associated Human Adenovirus Type 37 in Human Corneal Epithelial Cells.Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):50. doi: 10.1167/iovs.61.10.50. Invest Ophthalmol Vis Sci. 2020. PMID: 32852546 Free PMC article.
-
The role of Ca2+ in the injury of host cells during the schizogenic stage of E. tenella.Poult Sci. 2022 Jul;101(7):101916. doi: 10.1016/j.psj.2022.101916. Epub 2022 Apr 9. Poult Sci. 2022. PMID: 35523032 Free PMC article.
-
Unravelling bacterial virulence factors in yeast: From identification to the elucidation of their mechanisms of action.Arch Microbiol. 2024 Jun 15;206(7):303. doi: 10.1007/s00203-024-04023-2. Arch Microbiol. 2024. PMID: 38878203 Review.
-
Contribution of carbohydrate-related metabolism in Herpesvirus infections.Curr Res Microb Sci. 2023 May 17;4:100192. doi: 10.1016/j.crmicr.2023.100192. eCollection 2023. Curr Res Microb Sci. 2023. PMID: 37273578 Free PMC article.
References
-
- Rottner K and Stradal TE (2011) Actin dynamics and turnover in cell motility. Curr Opin Cell Biol 23, 569–578. - PubMed
-
- Rottner K, Faix J, Bogdan S, Linder S and Kerkhoff E (2017) Actin assembly mechanisms at a glance. J Cell Sci 130, 3427–3435. - PubMed
-
- Siton‐Mendelson O and Bernheim‐Groswasser A (2017) Functional actin networks under construction: the cooperative action of actin nucleation and elongation factors. Trends Biochem Sci 42, 414–430. - PubMed
-
- Derry JM, Ochs HD and Francke U (1994) Isolation of a novel gene mutated in Wiskott‐Aldrich syndrome. Cell 78, 635–644. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

