The Neuroregenerative Capacity of Olfactory Stem Cells Is Not Limitless: Implications for Aging
- PMID: 29934351
- PMCID: PMC6070664
- DOI: 10.1523/JNEUROSCI.3261-17.2018
The Neuroregenerative Capacity of Olfactory Stem Cells Is Not Limitless: Implications for Aging
Abstract
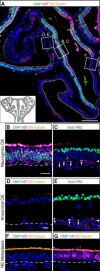
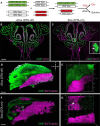
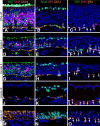
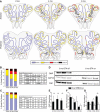
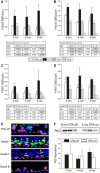
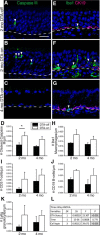
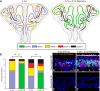
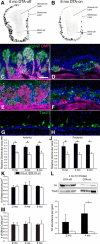
The olfactory epithelium (OE) of vertebrates is a highly regenerative neuroepithelium that is maintained under normal conditions by a population of stem and progenitor cells, globose basal cells (GBCs), which also contribute to epithelial reconstitution after injury. However, aging of the OE often leads to neurogenic exhaustion, the disappearance of both GBCs and olfactory sensory neurons (OSNs). Aneuronal tissue may remain as olfactory, with an uninterrupted sheet of apically arrayed microvillar-capped sustentacular cell, or may undergo respiratory metaplasia. We have generated a transgenic mouse model for neurogenic exhaustion using olfactory marker protein-driven Tet-off regulation of the A subunit of Diphtheria toxin such that the death of mature OSNs is accelerated. At as early as 2 months of age, the epithelium of transgenic mice, regardless of sex, recapitulates what is seen in the aged OE of humans and rodents. Areas of the epithelium completely lack neurons and GBCs; whereas the horizontal basal cells, a reserve stem cell population, show no evidence of activation. Surprisingly, other areas that were olfactory undergo respiratory metaplasia. The impact of accelerated neuronal death and reduced innervation on the olfactory bulb (OB) was also examined. Constant neuronal turnover leaves glomeruli shrunken and affects the dopaminergic interneurons in the periglomerular layer. Moreover, the acceleration of OSN death can be reversed in those areas where some GBCs persist. However, the projection onto the OB recovers incompletely and the reinnervated glomeruli are markedly altered. Therefore, the capacity for OE regeneration is tempered when GBCs disappear.SIGNIFICANCE STATEMENT A large percentage of humans lose or suffer a significant decline in olfactory function as they age. Therefore, quality of life suffers and safety and nutritional status are put at risk. With age, the OE apparently becomes incapable of fully maintaining the neuronal population of the epithelium despite its well known capacity for recovering from most forms of injury when younger. Efforts to identify the mechanism by which olfactory neurogenesis becomes exhausted with age require a powerful model for accelerating age-related tissue pathology. The current OMP-tTA;TetO-DTA transgenic mouse model, in which olfactory neurons die when they reach maturity and accelerated death can be aborted to assess the capacity for structural recovery, satisfies that need.
Keywords: aging; degeneration; neuroepithelium; olfactory; stem.
Copyright © 2018 the authors 0270-6474/18/386806-19$15.00/0.
Figures

Similar articles
-
Integrated age-related immunohistological changes occur in human olfactory epithelium and olfactory bulb.J Comp Neurol. 2022 Aug;530(12):2154-2175. doi: 10.1002/cne.25325. Epub 2022 Apr 9. J Comp Neurol. 2022. PMID: 35397118 Free PMC article.
-
Purinergic signalling selectively modulates maintenance but not repair neurogenesis in the zebrafish olfactory epithelium.FEBS J. 2020 Jul;287(13):2699-2722. doi: 10.1111/febs.15170. Epub 2019 Dec 29. FEBS J. 2020. PMID: 31821713
-
Global expression profiling of globose basal cells and neurogenic progression within the olfactory epithelium.J Comp Neurol. 2013 Mar 1;521(4):833-59. doi: 10.1002/cne.23204. J Comp Neurol. 2013. PMID: 22847514 Free PMC article.
-
Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license.J Comp Neurol. 2017 Mar 1;525(4):1034-1054. doi: 10.1002/cne.24105. Epub 2016 Sep 27. J Comp Neurol. 2017. PMID: 27560601 Free PMC article. Review.
-
Identification and molecular regulation of neural stem cells in the olfactory epithelium.Exp Cell Res. 2005 Jun 10;306(2):309-16. doi: 10.1016/j.yexcr.2005.03.027. Epub 2005 Apr 21. Exp Cell Res. 2005. PMID: 15925585 Review.
Cited by
-
[Future therapeutic strategies for olfactory disorders: electrical stimulation, stem cell therapy, and transplantation of olfactory epithelium-an overview].HNO. 2021 Aug;69(8):623-632. doi: 10.1007/s00106-021-01060-x. Epub 2021 May 14. HNO. 2021. PMID: 33988723 Free PMC article. Review. German.
-
Integrated age-related immunohistological changes occur in human olfactory epithelium and olfactory bulb.J Comp Neurol. 2022 Aug;530(12):2154-2175. doi: 10.1002/cne.25325. Epub 2022 Apr 9. J Comp Neurol. 2022. PMID: 35397118 Free PMC article.
-
Type 2 and Non-type 2 Inflammation in the Upper Airways: Cellular and Molecular Alterations in Olfactory Neuroepithelium Cell Populations.Curr Allergy Asthma Rep. 2024 Apr;24(4):211-219. doi: 10.1007/s11882-024-01137-x. Epub 2024 Mar 16. Curr Allergy Asthma Rep. 2024. PMID: 38492160 Free PMC article. Review.
-
Spanish Validation for Olfactory Function Testing Using the Sniffin' Sticks Olfactory Test: Threshold, Discrimination, and Identification.Brain Sci. 2020 Dec 7;10(12):943. doi: 10.3390/brainsci10120943. Brain Sci. 2020. PMID: 33297359 Free PMC article.
-
The Role of Knockout Olfactory Receptor Genes in Odor Discrimination.Genes (Basel). 2021 Apr 23;12(5):631. doi: 10.3390/genes12050631. Genes (Basel). 2021. PMID: 33922566 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
