The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification
- PMID: 29928541
- PMCID: PMC6002476
- DOI: 10.1038/s41413-018-0021-z
The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification
Abstract
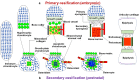
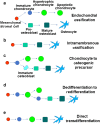
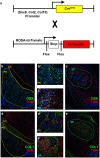
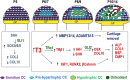
There is a worldwide epidemic of skeletal diseases causing not only a public health issue but also accounting for a sizable portion of healthcare expenditures. The vertebrate skeleton is known to be formed by mesenchymal cells condensing into tissue elements (patterning phase) followed by their differentiation into cartilage (chondrocytes) or bone (osteoblasts) cells within the condensations. During the growth and remodeling phase, bone is formed directly via intramembranous ossification or through a cartilage to bone conversion via endochondral ossification routes. The canonical pathway of the endochondral bone formation process involves apoptosis of hypertrophic chondrocytes followed by vascular invasion that brings in osteoclast precursors to remove cartilage and osteoblast precursors to form bone. However, there is now an emerging role for chondrocyte-to-osteoblast transdifferentiation in the endochondral ossification process. Although the concept of "transdifferentiation" per se is not recent, new data using a variety of techniques to follow the fate of chondrocytes in different bones during embryonic and post-natal growth as well as during fracture repair in adults have identified three different models for chondrocyte-to-osteoblast transdifferentiation (direct transdifferentiation, dedifferentiation to redifferentiation, and chondrocyte to osteogenic precursor). This review focuses on the emerging models of chondrocyte-to-osteoblast transdifferentiation and their implications for the treatment of skeletal diseases as well as the possible signaling pathways that contribute to chondrocyte-to-osteoblast transdifferentiation processes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
The Emerging Role of Cell Transdifferentiation in Skeletal Development and Diseases.Int J Mol Sci. 2022 May 26;23(11):5974. doi: 10.3390/ijms23115974. Int J Mol Sci. 2022. PMID: 35682655 Free PMC article. Review.
-
Chondrocyte-to-osteoblast transformation in mandibular fracture repair.J Orthop Res. 2021 Aug;39(8):1622-1632. doi: 10.1002/jor.24904. Epub 2020 Nov 18. J Orthop Res. 2021. PMID: 33140859 Free PMC article.
-
Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice.PLoS Genet. 2014 Dec 4;10(12):e1004820. doi: 10.1371/journal.pgen.1004820. eCollection 2014 Dec. PLoS Genet. 2014. PMID: 25474590 Free PMC article.
-
Bone tissue and histological and molecular events during development of the long bones.Ann Anat. 2021 May;235:151704. doi: 10.1016/j.aanat.2021.151704. Epub 2021 Feb 16. Ann Anat. 2021. PMID: 33600952 Review.
-
Comparative Study of DHA with Different Molecular Forms for Ameliorating Osteoporosis by Promoting Chondrocyte-to-Osteoblast Transdifferentiation in the Growth Plate of Ovariectomized Mice.J Agric Food Chem. 2021 Sep 15;69(36):10562-10571. doi: 10.1021/acs.jafc.1c03228. Epub 2021 Aug 31. J Agric Food Chem. 2021. PMID: 34464107
Cited by
-
Direct Reprogramming of Mouse Subchondral Bone Osteoblasts into Chondrocyte-like Cells.Biomedicines. 2022 Oct 14;10(10):2582. doi: 10.3390/biomedicines10102582. Biomedicines. 2022. PMID: 36289842 Free PMC article.
-
Moderate static magnetic field promotes fracture healing and regulates iron metabolism in mice.Biomed Eng Online. 2023 Nov 15;22(1):107. doi: 10.1186/s12938-023-01170-3. Biomed Eng Online. 2023. PMID: 37968671 Free PMC article.
-
TAZ is required for chondrogenesis and skeletal development.Cell Discov. 2021 Apr 20;7(1):26. doi: 10.1038/s41421-021-00254-5. Cell Discov. 2021. PMID: 33879790 Free PMC article.
-
Morphogenesis of the femur at different stages of normal human development.PLoS One. 2019 Aug 23;14(8):e0221569. doi: 10.1371/journal.pone.0221569. eCollection 2019. PLoS One. 2019. PMID: 31442281 Free PMC article.
-
MicroRNA-138: an emerging regulator of skeletal development, homeostasis, and disease.Am J Physiol Cell Physiol. 2023 Dec 1;325(6):C1387-C1400. doi: 10.1152/ajpcell.00382.2023. Epub 2023 Oct 16. Am J Physiol Cell Physiol. 2023. PMID: 37842749 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

