γδ T cells: an immunotherapeutic approach for HIV cure strategies
- PMID: 29925697
- PMCID: PMC6124426
- DOI: 10.1172/jci.insight.120121
γδ T cells: an immunotherapeutic approach for HIV cure strategies
Abstract
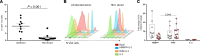
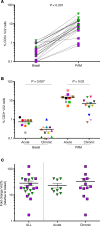
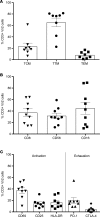
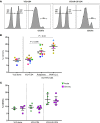
Current strategies aimed to cure HIV infection are based on combined efforts to reactivate the virus from latency and improve immune effector cell function to clear infected cells. These strategies are primarily focused on CD8+ T cells and approaches are challenging due to insufficient HIV antigen production from infected cells and poor HIV-specific CD8+ T cells. γδ T cells represent a unique subset of effector T cells that can traffic to tissues, and selectively target cancer or virally infected cells without requiring MHC presentation. We analyzed whether γδ T cells represent a complementary/alternative immunotherapeutic approach towards HIV cure strategies. γδ T cells from HIV-infected virologically suppressed donors were expanded with bisphosphonate pamidronate (PAM) and cells were used in autologous cellular systems ex vivo. These cells (a) are potent cytotoxic effectors able to efficiently inhibit HIV replication ex vivo, (b) degranulate in the presence of autologous infected CD4+ T cells, and (c) specifically clear latently infected cells after latency reversal with vorinostat. This is the first proof of concept to our knowledge showing that γδ T cells target and clear autologous HIV reservoirs upon latency reversal. Our results open potentially new insights into the immunotherapeutic use of γδ T cells for current interventions in HIV eradication strategies.
Keywords: AIDS/HIV; Immunotherapy.
Conflict of interest statement
Figures

Similar articles
-
Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo.J Virol. 2018 May 29;92(12):e00235-18. doi: 10.1128/JVI.00235-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29593039 Free PMC article.
-
Boosting the Immune System for HIV Cure: A γδ T Cell Perspective.Front Cell Infect Microbiol. 2020 May 19;10:221. doi: 10.3389/fcimb.2020.00221. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32509594 Free PMC article. Review.
-
Reactivation Kinetics of HIV-1 and Susceptibility of Reactivated Latently Infected CD4+ T Cells to HIV-1-Specific CD8+ T Cells.J Virol. 2015 Sep;89(18):9631-8. doi: 10.1128/JVI.01454-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178987 Free PMC article. Clinical Trial.
-
Toward T Cell-Mediated Control or Elimination of HIV Reservoirs: Lessons From Cancer Immunology.Front Immunol. 2019 Sep 10;10:2109. doi: 10.3389/fimmu.2019.02109. eCollection 2019. Front Immunol. 2019. PMID: 31552045 Free PMC article. Review.
-
Chimeric Antigen Receptor T Cells Guided by the Single-Chain Fv of a Broadly Neutralizing Antibody Specifically and Effectively Eradicate Virus Reactivated from Latency in CD4+ T Lymphocytes Isolated from HIV-1-Infected Individuals Receiving Suppressive Combined Antiretroviral Therapy.J Virol. 2016 Oct 14;90(21):9712-9724. doi: 10.1128/JVI.00852-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535056 Free PMC article.
Cited by
-
γδ T cells mediate robust anti-HIV functions during antiretroviral therapy regardless of immune checkpoint expression.Clin Transl Immunology. 2024 Jan 29;13(2):e1486. doi: 10.1002/cti2.1486. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38299190 Free PMC article.
-
Role of MHC class I pathways in Mycobacterium tuberculosis antigen presentation.Front Cell Infect Microbiol. 2023 Mar 15;13:1107884. doi: 10.3389/fcimb.2023.1107884. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37009503 Free PMC article. Review.
-
γδ T-cell responses during HIV infection and antiretroviral therapy.Clin Transl Immunology. 2019 Jul 17;8(7):e01069. doi: 10.1002/cti2.1069. eCollection 2019. Clin Transl Immunology. 2019. PMID: 31321033 Free PMC article. Review.
-
Emerging role of γδ T cells in vaccine-mediated protection from infectious diseases.Clin Transl Immunology. 2019 Aug 28;8(8):e1072. doi: 10.1002/cti2.1072. eCollection 2019. Clin Transl Immunology. 2019. PMID: 31485329 Free PMC article. Review.
-
HIV Latency in Myeloid Cells: Challenges for a Cure.Pathogens. 2022 May 24;11(6):611. doi: 10.3390/pathogens11060611. Pathogens. 2022. PMID: 35745465 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

