Platelets Enhance Dendritic Cell Responses against Staphylococcus aureus through CD40-CD40L
- PMID: 29914928
- PMCID: PMC6105897
- DOI: 10.1128/IAI.00186-18
Platelets Enhance Dendritic Cell Responses against Staphylococcus aureus through CD40-CD40L
Abstract
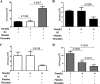
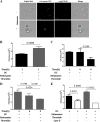
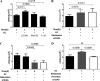
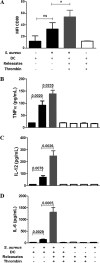
Staphylococcus aureus is a major human pathogen that can cause mild to severe life-threatening infections in many tissues and organs. Platelets are known to participate in protection against S. aureus by direct killing and by enhancing the activities of neutrophils and macrophages in clearing S. aureus infection. Platelets have also been shown to induce monocyte differentiation into dendritic cells and to enhance activation of dendritic cells. Therefore, in the present study, we explored the role of platelets in enhancing bone marrow-derived dendritic cell (BMDC) function against S. aureus We observed a significant increase in dendritic cell phagocytosis and intracellular killing of a methicillin-resistant Staphylococcus aureus (MRSA) strain (USA300) by thrombin-activated platelets or their releasates. Enhancement of bacterial uptake and killing by DCs is mediated by platelet-derived CD40L. Coculture of USA300 and BMDCs in the presence of thrombin-activated platelet releasates invokes upregulation of the maturation marker CD80 on DCs and enhanced production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin 12 (IL-12), and IL-6. Overall, these observations support our hypothesis that platelets play a critical role in the host defense against S. aureus infection. Platelets stimulate DCs, leading to direct killing of S. aureus and enhanced DC maturation, potentially leading to adaptive immune responses against S. aureus.
Keywords: CD40L; S. aureus; dendritic cells; phagocytosis; platelets.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Platelets Mediate Host Defense against Staphylococcus aureus through Direct Bactericidal Activity and by Enhancing Macrophage Activities.J Immunol. 2017 Jan 1;198(1):344-351. doi: 10.4049/jimmunol.1601178. Epub 2016 Nov 28. J Immunol. 2017. PMID: 27895175 Free PMC article.
-
PSM Peptides From Community-Associated Methicillin-Resistant Staphylococcus aureus Impair the Adaptive Immune Response via Modulation of Dendritic Cell Subsets in vivo.Front Immunol. 2019 May 10;10:995. doi: 10.3389/fimmu.2019.00995. eCollection 2019. Front Immunol. 2019. PMID: 31134074 Free PMC article.
-
Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro.J Immunol. 2004 May 1;172(9):5297-303. doi: 10.4049/jimmunol.172.9.5297. J Immunol. 2004. PMID: 15100268
-
Dendritic cells during Staphylococcus aureus infection: subsets and roles.J Transl Med. 2014 Dec 18;12:358. doi: 10.1186/s12967-014-0358-z. J Transl Med. 2014. PMID: 25519813 Free PMC article. Review.
-
How methicillin-resistant Staphylococcus aureus evade neutrophil killing.Curr Opin Hematol. 2015 Jan;22(1):30-5. doi: 10.1097/MOH.0000000000000096. Curr Opin Hematol. 2015. PMID: 25394313 Free PMC article. Review.
Cited by
-
Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy.Int J Mol Sci. 2022 Feb 19;23(4):2321. doi: 10.3390/ijms23042321. Int J Mol Sci. 2022. PMID: 35216433 Free PMC article. Review.
-
The Immune Nature of Platelets Revisited.Transfus Med Rev. 2020 Oct;34(4):209-220. doi: 10.1016/j.tmrv.2020.09.005. Epub 2020 Sep 19. Transfus Med Rev. 2020. PMID: 33051111 Free PMC article. Review.
-
Platelets interact with CD169+ macrophages and cDC1 and enhance liposome-induced CD8+ T cell responses.Front Immunol. 2023 Nov 20;14:1290272. doi: 10.3389/fimmu.2023.1290272. eCollection 2023. Front Immunol. 2023. PMID: 38054006 Free PMC article.
-
The Immunological Capacity of Thrombocytes.Int J Mol Sci. 2023 Aug 18;24(16):12950. doi: 10.3390/ijms241612950. Int J Mol Sci. 2023. PMID: 37629130 Free PMC article. Review.
-
Platelets and platelet-derived vesicles as an innovative cellular and subcellular platform for managing multiple sclerosis.Mol Biol Rep. 2023 May;50(5):4675-4686. doi: 10.1007/s11033-023-08322-7. Epub 2023 Apr 6. Mol Biol Rep. 2023. PMID: 37022526 Free PMC article. Review.
References
-
- Aslam R, Speck ER, Kim M, Crow AR, Bang KWA, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. 2006. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood 107:637–641. doi:10.1182/blood-2005-06-2202. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

