Impact of TGEV infection on the pig small intestine
- PMID: 29914507
- PMCID: PMC6006930
- DOI: 10.1186/s12985-018-1012-9
Impact of TGEV infection on the pig small intestine
Abstract
Background: Pig diarrhea causes high mortality and large economic losses in the swine industry. Transmissible gastroenteritis virus (TGEV) causes pig diarrhea, with 100% mortality in piglets less than 2 weeks old. No investigation has yet been made of the small intestine of piglets that survived infection by TGEV.
Methods: In this study, we evaluated the impact of TGEV infection on the small intestine of recovered pigs.
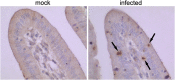
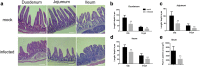
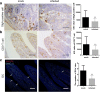
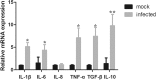
Results: Histological analyses showed that TGEV infection led to villi atrophy, and reduced villous height and crypt depth. The number of SIgA positive cells, CD3+T cells, and dendritic cells (DCs) in jejunum decreased after TGEV infection in vivo. In contrast, microfold cell (M cell) numbers and cell proliferation increased in infected pigs. TGEV infection also significantly enhanced the mRNA expression levels of cytokine IL-1β, IL-6, TNF-α, IL-10, and TGF-β. Additionally, lower gene copy numbers of Lactobacillus, and higher numbers of Enterobacteriaceae, were detected in mucosal scraping samples from TGEV-infected pigs.
Conclusions: TGEV infection damages the small intestine, impairs immune functions, and increases pathogenic bacterial loading, all of which may facilitate secondary infections by other pathogens. These findings help quantify the impact of TGEV infection and clarify the pathogenic mechanisms underlying its effects in pigs.
Keywords: Infection; Pig; Small intestine; Transmissible gastroenteritis virus.
Conflict of interest statement
Ethics approval
The animal experiments were approved by the regulations and guidelines of laboratory animals of Nanjing Agriculture University (Nanjing, China).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Persistent Transmissible Gastroenteritis Virus Infection Enhances Enterotoxigenic Escherichia coli K88 Adhesion by Promoting Epithelial-Mesenchymal Transition in Intestinal Epithelial Cells.J Virol. 2017 Oct 13;91(21):e01256-17. doi: 10.1128/JVI.01256-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28794036 Free PMC article.
-
Transmissible gastroenteritis virus infection decreases arginine uptake by downregulating CAT-1 expression.Vet Res. 2018 Sep 20;49(1):95. doi: 10.1186/s13567-018-0591-1. Vet Res. 2018. PMID: 30236161 Free PMC article.
-
Effects of virulent and attenuated transmissible gastroenteritis virus on the ability of porcine dendritic cells to sample and present antigen.Vet Microbiol. 2014 Jun 25;171(1-2):74-86. doi: 10.1016/j.vetmic.2014.03.017. Epub 2014 Mar 27. Vet Microbiol. 2014. PMID: 24742951 Free PMC article.
-
Prevalence of transmissible gastroenteritis among swine populations in China during 1983-2022: A systematic review and meta-analysis.Microb Pathog. 2023 Oct;183:106320. doi: 10.1016/j.micpath.2023.106320. Epub 2023 Aug 23. Microb Pathog. 2023. PMID: 37625663 Review.
-
Transmissible gastroenteritis virus infection: a vanishing specter.Dtsch Tierarztl Wochenschr. 2006 Apr;113(4):157-9. Dtsch Tierarztl Wochenschr. 2006. PMID: 16716052 Review.
Cited by
-
Dietary milk fat globule membrane supplementation during late gestation increased the growth of neonatal piglets by improving their plasma parameters, intestinal barriers, and fecal microbiota.RSC Adv. 2020 Apr 30;10(29):16987-16998. doi: 10.1039/d0ra02618b. eCollection 2020 Apr 29. RSC Adv. 2020. PMID: 35521473 Free PMC article.
-
EIF4A2 interacts with the membrane protein of transmissible gastroenteritis coronavirus and plays a role in virus replication.Res Vet Sci. 2019 Apr;123:39-46. doi: 10.1016/j.rvsc.2018.12.005. Epub 2018 Dec 17. Res Vet Sci. 2019. PMID: 30583231 Free PMC article.
-
Non-coding RNAs derived from the foot-and-mouth disease virus genome trigger broad antiviral activity against coronaviruses.Front Immunol. 2023 Mar 29;14:1166725. doi: 10.3389/fimmu.2023.1166725. eCollection 2023. Front Immunol. 2023. PMID: 37063925 Free PMC article.
-
Decellularized small intestine scaffolds: a potential xenograft for restoration of intestinal perforation.Tissue Barriers. 2024 Oct;12(4):2290940. doi: 10.1080/21688370.2023.2290940. Epub 2023 Dec 5. Tissue Barriers. 2024. PMID: 38053224 Free PMC article.
-
HSP90AB1 Is a Host Factor Required for Transmissible Gastroenteritis Virus Infection.Int J Mol Sci. 2023 Nov 4;24(21):15971. doi: 10.3390/ijms242115971. Int J Mol Sci. 2023. PMID: 37958953 Free PMC article.
References
-
- Jacobson M. Enteric diseases in pigs from weaning to slaughter. Swedish University of Agricultural Sciences, Department of Large Animal Clinical Sciences. 2003.
-
- Lawhorn B. Diarrheal disease in show swine. Texas Agricultural Extension Service, Texas A & M University System. 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

