A conserved role for Syntaxin-1 in pre- and post-commissural midline axonal guidance in fly, chick, and mouse
- PMID: 29912942
- PMCID: PMC6029812
- DOI: 10.1371/journal.pgen.1007432
A conserved role for Syntaxin-1 in pre- and post-commissural midline axonal guidance in fly, chick, and mouse
Abstract
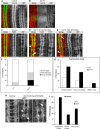
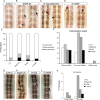
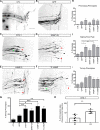
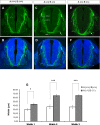
Axonal growth and guidance rely on correct growth cone responses to guidance cues. Unlike the signaling cascades that link axonal growth to cytoskeletal dynamics, little is known about the crosstalk mechanisms between guidance and membrane dynamics and turnover. Recent studies indicate that whereas axonal attraction requires exocytosis, chemorepulsion relies on endocytosis. Indeed, our own studies have shown that Netrin-1/Deleted in Colorectal Cancer (DCC) signaling triggers exocytosis through the SNARE Syntaxin-1 (STX1). However, limited in vivo evidence is available about the role of SNARE proteins in axonal guidance. To address this issue, here we systematically deleted SNARE genes in three species. We show that loss-of-function of STX1 results in pre- and post-commissural axonal guidance defects in the midline of fly, chick, and mouse embryos. Inactivation of VAMP2, Ti-VAMP, and SNAP25 led to additional abnormalities in axonal guidance. We also confirmed that STX1 loss-of-function results in reduced sensitivity of commissural axons to Slit-2 and Netrin-1. Finally, genetic interaction studies in Drosophila show that STX1 interacts with both the Netrin-1/DCC and Robo/Slit pathways. Our data provide evidence of an evolutionarily conserved role of STX1 and SNARE proteins in midline axonal guidance in vivo, by regulating both pre- and post-commissural guidance mechanisms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A signaling mechanism coupling netrin-1/deleted in colorectal cancer chemoattraction to SNARE-mediated exocytosis in axonal growth cones.J Neurosci. 2011 Oct 12;31(41):14463-80. doi: 10.1523/JNEUROSCI.3018-11.2011. J Neurosci. 2011. PMID: 21994363 Free PMC article.
-
SNARE proteins play a role in motor axon guidance in vertebrates and invertebrates.Dev Neurobiol. 2017 Sep;77(8):963-974. doi: 10.1002/dneu.22481. Epub 2017 Feb 12. Dev Neurobiol. 2017. PMID: 28033683
-
Netrin1-DCC-Mediated Attraction Guides Post-Crossing Commissural Axons in the Hindbrain.J Neurosci. 2015 Aug 19;35(33):11707-18. doi: 10.1523/JNEUROSCI.0613-15.2015. J Neurosci. 2015. PMID: 26290247 Free PMC article.
-
Axon guidance at the midline choice point.Dev Dyn. 2001 Jun;221(2):154-81. doi: 10.1002/dvdy.1143. Dev Dyn. 2001. PMID: 11376484 Review.
-
[Netrin-1 and axonal guidance: signaling and asymmetrical translation].Med Sci (Paris). 2007 Mar;23(3):311-5. doi: 10.1051/medsci/2007233311. Med Sci (Paris). 2007. PMID: 17349294 Review. French.
Cited by
-
Syntaxin-1 is necessary for UNC5A-C/Netrin-1-dependent macropinocytosis and chemorepulsion.Front Mol Neurosci. 2023 Sep 27;16:1253954. doi: 10.3389/fnmol.2023.1253954. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37829513 Free PMC article.
-
Pre- and post-synaptic roles for DCC in memory consolidation in the adult mouse hippocampus.Mol Brain. 2020 Apr 7;13(1):56. doi: 10.1186/s13041-020-00597-2. Mol Brain. 2020. PMID: 32264905 Free PMC article.
-
Molecular basis of the functions of the mammalian neuronal growth cone revealed using new methods.Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(7):358-377. doi: 10.2183/pjab.95.026. Proc Jpn Acad Ser B Phys Biol Sci. 2019. PMID: 31406059 Free PMC article. Review.
-
Syntaxin-1/TI-VAMP SNAREs interact with Trk receptors and are required for neurotrophin-dependent outgrowth.Oncotarget. 2018 Nov 13;9(89):35922-35940. doi: 10.18632/oncotarget.26307. eCollection 2018 Nov 13. Oncotarget. 2018. PMID: 30542508 Free PMC article.
-
Optimizing Efficient RNAi-Mediated Control of Hemipteran Pests (Psyllids, Leafhoppers, Whitefly): Modified Pyrimidines in dsRNA Triggers.Plants (Basel). 2021 Aug 26;10(9):1782. doi: 10.3390/plants10091782. Plants (Basel). 2021. PMID: 34579315 Free PMC article.
References
-
- Del Rio JA, Gonzalez-Billault C, Urena JM, Jimenez EM, Barallobre MJ, et al. (2004) MAP1B is required for Netrin 1 signaling in neuronal migration and axonal guidance. Curr Biol 14: 840–850. doi: 10.1016/j.cub.2004.04.046 - DOI - PubMed
-
- Nicol X, Hong KP, Spitzer NC (2011) Spatial and temporal second messenger codes for growth cone turning. Proc Natl Acad Sci U S A 108: 13776–13781. doi: 10.1073/pnas.1100247108 - DOI - PMC - PubMed
-
- Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG (2010) Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 141: 632–644. doi: 10.1016/j.cell.2010.04.008 - DOI - PMC - PubMed
-
- Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, et al. (2008) DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 133: 1241–1254. doi: 10.1016/j.cell.2008.05.030 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

