Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post-inflammatory rat model for irritable bowel syndrome
- PMID: 29911328
- PMCID: PMC6086981
- DOI: 10.1111/bph.14396
Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post-inflammatory rat model for irritable bowel syndrome
Abstract
Background and purpose: Serine proteases have been re suggested as important mediators of visceral pain. We investigated their effect by using newly developed serine protease inhibitors with a well-characterized inhibitory profile in a rat model of post-inflammatory irritable bowel syndrome (IBS).
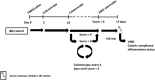
Experimental approach: Colitis was induced in rats receiving intrarectal trinitrobenzenesulphonic acid; controls received 0.9% NaCl. Colonoscopies were performed on day 3, to confirm colitis, and later until mucosal healing. Visceral hypersensitivity was quantified by visceromotor responses (VMRs) to colorectal distension, 30 min after i.p. injection of the serine protease inhibitors nafamostat, UAMC-00050 or UAMC-01162. Serine proteases, protease-activated receptors (PARs) and TRP channels were quantified by qPCR and immunohistochemistry. Proteolytic activity was characterized using fluorogenic substrates.
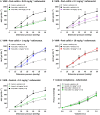
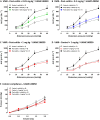
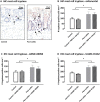
Key results: VMR was significantly elevated in post-colitis rats. Nafamostat normalized VMRs at the lowest dose tested. UAMC-00050 and UAMC-01162 significantly decreased VMR dose-dependently. Expression of mRNA for tryptase-αβ-1and PAR4, and tryptase immunoreactivity was significantly increased in the colon of post-colitis animals. Trypsin-like activity was also significantly increased in the colon but not in the faeces. PAR2 and TRPA1 immunoreactivity co-localized with CGRP-positive nerve fibres in control and post-colitis animals.
Conclusions and implications: Increased expression of serine proteases and activity together with increased expression of downstream molecules at the colonic and DRG level and in CGRP-positive sensory nerve fibres imply a role for serine proteases in post-inflammatory visceral hypersensitivity. Our results support further investigation of serine protease inhibitors as an interesting treatment strategy for IBS-related visceral pain.
© 2018 The British Pharmacological Society.
Figures




Similar articles
-
The Effect of Serine Protease Inhibitors on Visceral Pain in Different Rodent Models With an Intestinal Insult.Front Pharmacol. 2022 Jun 2;13:765744. doi: 10.3389/fphar.2022.765744. eCollection 2022. Front Pharmacol. 2022. PMID: 35721192 Free PMC article.
-
Local Colonic Administration of a Serine Protease Inhibitor Improves Post-Inflammatory Visceral Hypersensitivity in Rats.Pharmaceutics. 2021 May 29;13(6):811. doi: 10.3390/pharmaceutics13060811. Pharmaceutics. 2021. PMID: 34072320 Free PMC article.
-
Chitin-glucan improves important pathophysiological features of irritable bowel syndrome.World J Gastroenterol. 2024 Apr 28;30(16):2258-2271. doi: 10.3748/wjg.v30.i16.2258. World J Gastroenterol. 2024. PMID: 38690023 Free PMC article.
-
Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: The role of proteases.World J Gastroenterol. 2016 Dec 21;22(47):10275-10286. doi: 10.3748/wjg.v22.i47.10275. World J Gastroenterol. 2016. PMID: 28058009 Free PMC article. Review.
-
Protease activated receptor 2: a new target for IBS treatment.Eur Rev Med Pharmacol Sci. 2008 Aug;12 Suppl 1:95-102. Eur Rev Med Pharmacol Sci. 2008. PMID: 18924448 Review.
Cited by
-
Proteolytic Cleavage of Bioactive Peptides and Protease-Activated Receptors in Acute and Post-Colitis.Int J Mol Sci. 2021 Oct 2;22(19):10711. doi: 10.3390/ijms221910711. Int J Mol Sci. 2021. PMID: 34639054 Free PMC article.
-
Understanding neuroimmune interactions in disorders of gut-brain interaction: from functional to immune-mediated disorders.Gut. 2023 Apr;72(4):787-798. doi: 10.1136/gutjnl-2020-320633. Epub 2023 Jan 19. Gut. 2023. PMID: 36657961 Free PMC article. Review.
-
Potential Roles of Enterochromaffin Cells in Early Life Stress-Induced Irritable Bowel Syndrome.Front Cell Neurosci. 2022 Mar 15;16:837166. doi: 10.3389/fncel.2022.837166. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35370559 Free PMC article. Review.
-
Editorial: Serine protease inhibitors and their therapeutic applications.Front Chem. 2022 Aug 8;10:980152. doi: 10.3389/fchem.2022.980152. eCollection 2022. Front Chem. 2022. PMID: 36003619 Free PMC article. No abstract available.
-
The emerging roles of bacterial proteases in intestinal diseases.Gut Microbes. 2023 Jan-Dec;15(1):2181922. doi: 10.1080/19490976.2023.2181922. Gut Microbes. 2023. PMID: 36843008 Free PMC article. Review.
References
-
- Annahazi A, Dabek M, Gecse K, Salvador‐Cartier C, Polizzi A, Rosztoczy A et al (2012). Proteinase‐activated receptor‐4 evoked colorectal analgesia in mice: an endogenously activated feed‐back loop in visceral inflammatory pain. Neurogastroenterol Motil 24: 76–85, e13. - PubMed
-
- Annahazi A, Ferrier L, Bezirard V, Leveque M, Eutamene H, Ait‐Belgnaoui A et al (2013). Luminal cysteine‐proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation‐predominant IBS. Am J Gastroenterol 108: 1322–1331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

