Prucalopride Inhibits Proliferation of Ovarian Cancer Cells via Phosphatidylinositol 3-Kinase (PI3K) Signaling Pathway
- PMID: 29909423
- PMCID: PMC6036960
- DOI: 10.12659/MSM.907853
Prucalopride Inhibits Proliferation of Ovarian Cancer Cells via Phosphatidylinositol 3-Kinase (PI3K) Signaling Pathway
Abstract
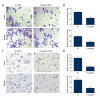
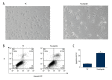
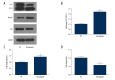
BACKGROUND Ovarian cancer is the second most common malignant tumor of the female reproductive system and is the leading cause of death of gynecological malignancies, but at present there is no effective and safe therapy. There is no previously published report on the anti-cancer effect of prucalopride, which is a high-affinity 5-HT4 receptor. The aim of the present study was to determine whether prucalopride can inhibit proliferation of ovarian cancer cells. MATERIAL AND METHODS The cell viability was detected by use of the Cell Counting Kit-8 (CCK-8) assay. The invasion and migration of SKOV3 and OVCAR3 cells was detected by Transwell assay. The cell apoptosis was detected by apoptosis flow detection and Caspase-Glo 3/7 Assay Systems. The apoptosis-related proteins, autophagy marker proteins, and the related-factors of phosphatidylinositol 3-kinase (PI3K) were detected by Western blot. RESULTS The CCK-8 proliferation test showed that prucalopride inhibited the growth of ovarian cancer cell lines SKOV3 and OVCAR3. In the Transwell assay, prucalopride inhibited cell invasion and migration. Furthermore, we found the expression of anti-apoptotic protein Bcl-2 decreased, whereas the expression of pro-apoptotic protein Caspase3 and Bax increased in the SKOV3 cell line treated with prucalopride, as well as cleaved PARP. In addition, the expression of p-AKT, p-mTOR, and p70S6K decreased in the prucalopride-treated group, and the expression of autophagy marker protein LC3-II/I and Beclin1 significantly increased, whereas the expression of p62 protein decreased. CONCLUSIONS The present study reveals that in ovarian cancer cells, prucalopride inhibits proliferation, migration, and invasion, and induces apoptosis and autophagy, which may be regulated by the PI3K signaling pathway. These results suggest prucalopride has potential as a new drug for clinical ovarian cancer treatment.
Conflict of interest statement
None.
Figures



Similar articles
-
Prucalopride inhibits the glioma cells proliferation and induces autophagy via AKT-mTOR pathway.BMC Neurol. 2018 Jun 4;18(1):80. doi: 10.1186/s12883-018-1083-7. BMC Neurol. 2018. PMID: 29866060 Free PMC article.
-
Endoplasmic reticulum stress promotes autophagy and apoptosis and reverses chemoresistance in human ovarian cancer cells.Oncotarget. 2017 Jul 25;8(30):49380-49394. doi: 10.18632/oncotarget.17673. Oncotarget. 2017. PMID: 28537902 Free PMC article.
-
[Effect of MTRR gene on apoptosis and autophagy pathways in multiresistant epithelial ovarian cancer].Zhonghua Fu Chan Ke Za Zhi. 2016 Apr 25;51(4):285-92. doi: 10.3760/cma.j.issn.0529-567X.2016.04.008. Zhonghua Fu Chan Ke Za Zhi. 2016. PMID: 27116987 Chinese.
-
Lysine-Specific Demethylase 1 (LSD1) Inhibitor S2101 Induces Autophagy via the AKT/mTOR Pathway in SKOV3 Ovarian Cancer Cells.Med Sci Monit. 2016 Dec 3;22:4742-4748. doi: 10.12659/msm.898825. Med Sci Monit. 2016. PMID: 27914215 Free PMC article.
-
Mitochondrial integration and ovarian cancer chemotherapy resistance.Exp Cell Res. 2021 Apr 15;401(2):112549. doi: 10.1016/j.yexcr.2021.112549. Epub 2021 Feb 26. Exp Cell Res. 2021. PMID: 33640393 Review.
Cited by
-
Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage.Drug Des Devel Ther. 2019 Apr 17;13:1187-1196. doi: 10.2147/DDDT.S196172. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31114159 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

