Defective cholesterol metabolism in haematopoietic stem cells promotes monocyte-driven atherosclerosis in rheumatoid arthritis
- PMID: 29905812
- PMCID: PMC6001889
- DOI: 10.1093/eurheartj/ehy119
Defective cholesterol metabolism in haematopoietic stem cells promotes monocyte-driven atherosclerosis in rheumatoid arthritis
Abstract
Aim: Rheumatoid arthritis (RA) is associated with an approximately two-fold elevated risk of cardiovascular (CV)-related mortality. Patients with RA present with systemic inflammation including raised circulating myeloid cells, but fail to display traditional CV risk-factors, particularly dyslipidaemia. We aimed to explore if increased circulating myeloid cells is associated with impaired atherosclerotic lesion regression or altered progression in RA.
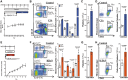
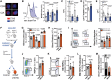
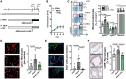
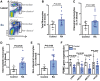
Methods and results: Using flow cytometry, we noted prominent monocytosis, neutrophilia, and thrombocytosis in two mouse models of RA. This was due to enhanced proliferation of the haematopoietic stem and progenitor cells (HSPCs) in the bone marrow and the spleen. HSPCs expansion was associated with an increase in the cholesterol content, due to a down-regulation of cholesterol efflux genes, Apoe, Abca1, and Abcg1. The HSPCs also had enhanced expression of key myeloid promoting growth factor receptors. Systemic inflammation was found to cause defective cellular cholesterol metabolism. Increased myeloid cells in mice with RA were associated with a significant impairment in lesion regression, even though cholesterol levels were equivalent to non-arthritic mice. Lesions from arthritic mice exhibited a less stable phenotype as demonstrated by increased immune cell infiltration, lipid accumulation, and decreased collagen formation. In a progression model, we noted monocytosis, enhanced monocytes recruitment to lesions, and increased plaque macrophages. This was reversed with administration of reconstituted high-density lipoprotein (rHDL). Furthermore, RA patients have expanded CD16+ monocyte subsets and a down-regulation of ABCA1 and ABCG1.
Conclusion: Rheumatoid arthritis impairs atherosclerotic regression and alters progression, which is associated with an expansion of myeloid cells and disturbed cellular cholesterol handling, independent of plasma cholesterol levels. Infusion of rHDL prevented enhanced myelopoiesis and monocyte entry into lesions. Targeting cellular cholesterol defects in people with RA, even if plasma cholesterol is within the normal range, may limit vascular disease.
Figures



Comment in
-
Is defective cholesterol efflux an integral inflammatory component in myelopoiesis-driven cardiovascular diseases?Eur Heart J. 2018 Jun 14;39(23):2168-2171. doi: 10.1093/eurheartj/ehy269. Eur Heart J. 2018. PMID: 29771312 No abstract available.
Similar articles
-
ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice.J Clin Invest. 2011 Oct;121(10):4138-49. doi: 10.1172/JCI57559. J Clin Invest. 2011. PMID: 21968112 Free PMC article.
-
Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency.Arterioscler Thromb Vasc Biol. 2014 May;34(5):976-84. doi: 10.1161/ATVBAHA.113.303097. Epub 2014 Mar 20. Arterioscler Thromb Vasc Biol. 2014. PMID: 24651678 Free PMC article.
-
Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis.Circulation. 2018 Aug 28;138(9):898-912. doi: 10.1161/CIRCULATIONAHA.117.032636. Circulation. 2018. PMID: 29588315 Free PMC article.
-
Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis.Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):2547-52. doi: 10.1161/ATVBAHA.112.300134. Arterioscler Thromb Vasc Biol. 2012. PMID: 23077140 Review.
-
The role of monocytosis and neutrophilia in atherosclerosis.J Cell Mol Med. 2018 Mar;22(3):1366-1382. doi: 10.1111/jcmm.13462. Epub 2018 Jan 24. J Cell Mol Med. 2018. PMID: 29364567 Free PMC article. Review.
Cited by
-
Trained Immunity: An Underlying Driver of Inflammatory Atherosclerosis.Front Immunol. 2020 Feb 21;11:284. doi: 10.3389/fimmu.2020.00284. eCollection 2020. Front Immunol. 2020. PMID: 32153588 Free PMC article. Review.
-
Transient Intermittent Hyperglycemia Accelerates Atherosclerosis by Promoting Myelopoiesis.Circ Res. 2020 Sep 11;127(7):877-892. doi: 10.1161/CIRCRESAHA.120.316653. Epub 2020 Jun 22. Circ Res. 2020. PMID: 32564710 Free PMC article.
-
Association of cumulative monocyte to high-density lipoprotein ratio with the risk of type 2 diabetes: a prospective cohort study.Cardiovasc Diabetol. 2022 Dec 3;21(1):268. doi: 10.1186/s12933-022-01701-7. Cardiovasc Diabetol. 2022. PMID: 36463212 Free PMC article.
-
Glycolysis Is Required for LPS-Induced Activation and Adhesion of Human CD14+CD16- Monocytes.Front Immunol. 2019 Sep 6;10:2054. doi: 10.3389/fimmu.2019.02054. eCollection 2019. Front Immunol. 2019. PMID: 31555280 Free PMC article.
-
Cardiovascular Disease Risk in Older Adults and Elderly Patients with Rheumatoid Arthritis: What Role Can Disease-Modifying Antirheumatic Drugs Play in Cardiovascular Risk Reduction?Drugs Aging. 2019 Jun;36(6):493-510. doi: 10.1007/s40266-019-00653-0. Drugs Aging. 2019. PMID: 30953327 Review.
References
-
- Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay MA.. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine 2003;82:407–413. - PubMed
-
- McInnes IB, Schett G.. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–2219. - PubMed
-
- Meune C, Touze E, Trinquart L, Allanore Y.. High risk of clinical cardiovascular events in rheumatoid arthritis: levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch Cardiovasc Dis 2010;103:253–261. - PubMed
-
- Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL, Kvien TK, Dougados M, Radner H, Atzeni F, Primdahl J, Södergren A, Wallberg Jonsson S, van Rompay J, Zabalan C, Pedersen TR, Jacobsson L, de Vlam K, Gonzalez-Gay MA, Semb AG, Kitas GD, Smulders YM, Szekanecz Z, Sattar N, Symmons DPM, Nurmohamed MT.. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

