A crystal structure of coil 1B of vimentin in the filamentous form provides a model of a high-order assembly of a vimentin filament
- PMID: 29905014
- PMCID: PMC8022333
- DOI: 10.1111/febs.14585
A crystal structure of coil 1B of vimentin in the filamentous form provides a model of a high-order assembly of a vimentin filament
Abstract
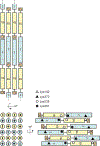
Vimentin is an intermediate filament (IF) protein that is expressed in leukocytes, fibroblasts and endothelial cells of blood vessels. Vimentin filaments contribute to structural stability of the cell membrane, organelle positioning and protein transport. Vimentin self-assembles into a dimer that subsequently forms high-order structures, including tetramers and octamers. The details of IF assembly at crystallographic resolutions are limited to the tetrameric form. We describe a crystal structure of a fragment of a vimentin rod domain (coil 1B) with a dimer of tetramers in the asymmetric unit. Coil 1B in the crystal is in an infinitely high-order filamentous assembly state, in which the tetramers are packed against each other laterally in an antiparallel fashion across the crystal lattice. In one of the directions of lateral packing, the tetramers pack against each other strictly head-to-tail, and in the orthogonal direction the tetramers pack in a staggered manner. This organization of the tetramers of coil 1B in the crystal lattice, together with previously reported biochemical and structural data, yield a model of high-order vimentin filament assembly.
Database: Structural data are available in the PDB under the accession number 5WHF.
Keywords: coiled-coil; crystal structure; helical domain; intermediate filament; oligomerization.
© 2018 Federation of European Biochemical Societies.
Figures
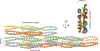
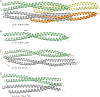


Similar articles
-
Assembly Kinetics of Vimentin Tetramers to Unit-Length Filaments: A Stopped-Flow Study.Biophys J. 2018 May 22;114(10):2408-2418. doi: 10.1016/j.bpj.2018.04.032. Epub 2018 May 10. Biophys J. 2018. PMID: 29754715 Free PMC article.
-
Atomic structure of the vimentin central α-helical domain and its implications for intermediate filament assembly.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13620-5. doi: 10.1073/pnas.1206836109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869704 Free PMC article.
-
Vimentin coil 1A-A molecular switch involved in the initiation of filament elongation.J Mol Biol. 2009 Jul 10;390(2):245-61. doi: 10.1016/j.jmb.2009.04.067. Epub 2009 May 5. J Mol Biol. 2009. PMID: 19422834
-
Molecular architecture of intermediate filaments.Bioessays. 2003 Mar;25(3):243-51. doi: 10.1002/bies.10246. Bioessays. 2003. PMID: 12596228 Review.
-
Deciphering vimentin assembly: Bridging theoretical models and experimental approaches.Mol Cells. 2024 Jul;47(7):100080. doi: 10.1016/j.mocell.2024.100080. Epub 2024 Jun 11. Mol Cells. 2024. PMID: 38871297 Free PMC article. Review.
Cited by
-
Molecular Docking of SP40 Peptide towards Cellular Receptors for Enterovirus 71 (EV-A71).Molecules. 2021 Oct 30;26(21):6576. doi: 10.3390/molecules26216576. Molecules. 2021. PMID: 34770987 Free PMC article.
-
Molecular Modeling of Pathogenic Mutations in the Keratin 1B Domain.Int J Mol Sci. 2020 Sep 10;21(18):6641. doi: 10.3390/ijms21186641. Int J Mol Sci. 2020. PMID: 32927888 Free PMC article.
-
Structure-Function Analyses of a Keratin Heterotypic Complex Identify Specific Keratin Regions Involved in Intermediate Filament Assembly.Structure. 2020 Mar 3;28(3):355-362.e4. doi: 10.1016/j.str.2020.01.002. Epub 2020 Jan 28. Structure. 2020. PMID: 31995743 Free PMC article.
-
Type III intermediate filaments in redox interplay: key role of the conserved cysteine residue.Biochem Soc Trans. 2024 Apr 24;52(2):849-860. doi: 10.1042/BST20231059. Biochem Soc Trans. 2024. PMID: 38451193 Free PMC article. Review.
-
Redox Regulation of PPARγ in Polarized Macrophages.PPAR Res. 2020 Jul 1;2020:8253831. doi: 10.1155/2020/8253831. eCollection 2020. PPAR Res. 2020. PMID: 32695149 Free PMC article.
References
-
- Herrmann H, Bar H, Kreplak L, Strelkov SV & Aebi U (2007) Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8, 562–573. - PubMed
-
- Steinert PM & Roop DR (1988) Molecular and cellular biology of intermediate filaments. Annu Rev Biochem 57, 593–625. - PubMed
-
- Albers K & Fuchs E (1992) The molecular biology of intermediate filament proteins. Int Rev Cytol 134, 243–279. - PubMed
-
- Lendahl U, Zimmerman LB & McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60, 585–595. - PubMed
-
- Herrmann H, Haner M, Brettel M, Muller SA, Goldie KN, Fedtke B, Lustig A, Franke WW & Aebi U (1996) Structure and assembly properties of the intermediate filament protein vimentin: the role of its head, rod and tail domains. J Mol Biol 264, 933–953. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

