Contrasting Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening Under Commercial Insurance vs. Medicare
- PMID: 29904156
- PMCID: PMC6768591
- DOI: 10.1038/s41395-018-0106-8
Contrasting Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening Under Commercial Insurance vs. Medicare
Abstract
Objectives: Most cost-effectiveness analyses of colorectal cancer (CRC) screening assume Medicare payment rates and a lifetime horizon. Our aims were to examine the implications of differential payment levels and time horizons for commercial insurers vs. Medicare on the cost-effectiveness of CRC screening.
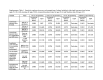
Methods: We used our validated Markov cohort simulation of CRC screening in the average risk US population to examine CRC screening at ages 50-64 under commercial insurance, and at ages 65-80 under Medicare, using a health-care sector perspective. Model outcomes included discounted quality-adjusted life-years (QALYs) and costs per person, and incremental cost/QALY gained.
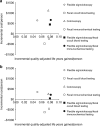
Results: Lifetime costs/person were 20-44% higher when assuming commercial payment rates rather than Medicare rates for people under 65. Most of the substantial clinical benefit of screening at ages 50-64 was realized at ages ≥65. For commercial payers with a time horizon of ages 50-64, fecal occult blood testing (FOBT) and fecal immunochemical testing (FIT) were cost-effective (<$61,000/QALY gained), but colonoscopy was costly (>$185,000/QALY gained). Medicare experienced substantial clinical benefits and cost-savings from screening done at ages <65, even if screening was not continued. Among those previously screened, continuing FOBT and FIT under Medicare was cost-saving and continuing colonoscopy was highly cost-effective (<$30,000/QALY gained), and initiating any screening in those previously unscreened was highly effective and cost-saving.
Conclusions: Modeling suggests that CRC screening is highly cost-effective over a lifetime even when considering higher payment rates by commercial payers vs. Medicare. Screening may appear relatively costly for commercial payers if only a time horizon of ages 50-64 is considered, but it is predicted to yield substantial clinical and economic benefits that accrue primarily at ages ≥65 under Medicare.
Figures


















Comment in
-
Importance of Age-Specific Insurer Perspective on Lifetime Cost Effectiveness of Colorectal Cancer Screening.Am J Gastroenterol. 2018 Dec;113(12):1754-1756. doi: 10.1038/s41395-018-0386-z. Epub 2018 Oct 29. Am J Gastroenterol. 2018. PMID: 30374119 Free PMC article.
Similar articles
-
Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia.Gastroenterology. 2016 Sep;151(3):427-439.e6. doi: 10.1053/j.gastro.2016.06.003. Epub 2016 Jun 14. Gastroenterology. 2016. PMID: 27311556
-
Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies.Am J Gastroenterol. 2013 Jan;108(1):120-32. doi: 10.1038/ajg.2012.380. Epub 2012 Dec 18. Am J Gastroenterol. 2013. PMID: 23247579
-
Cost-effectiveness and budget impact analyses of colorectal cancer screenings in a low- and middle-income country: example from Thailand.J Med Econ. 2019 Dec;22(12):1351-1361. doi: 10.1080/13696998.2019.1674065. Epub 2019 Oct 12. J Med Econ. 2019. PMID: 31560247
-
Impact of comorbidity on colorectal cancer screening cost-effectiveness study in diabetic populations.J Gen Intern Med. 2012 Jun;27(6):730-8. doi: 10.1007/s11606-011-1972-6. Epub 2012 Jan 12. J Gen Intern Med. 2012. PMID: 22237663 Free PMC article. Review.
-
A Comparison of the Cost-Effectiveness of Fecal Occult Blood Tests with Different Test Characteristics in the Context of Annual Screening in the Medicare Population [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2003 Aug 9. Rockville (MD): Agency for Healthcare Research and Quality (US); 2003 Aug 9. PMID: 25905156 Free Books & Documents. Review.
Cited by
-
Cost-Effectiveness of Risk-Stratified Colorectal Cancer Screening Based on Polygenic Risk: Current Status and Future Potential.JNCI Cancer Spectr. 2019 Oct 14;4(1):pkz086. doi: 10.1093/jncics/pkz086. eCollection 2020 Feb. JNCI Cancer Spectr. 2019. PMID: 32025627 Free PMC article.
-
Is there a place for sigmoidoscopy in colorectal cancer screening? A systematic review and critical appraisal of cost-effectiveness models.PLoS One. 2023 Aug 18;18(8):e0290353. doi: 10.1371/journal.pone.0290353. eCollection 2023. PLoS One. 2023. PMID: 37594967 Free PMC article.
-
Importance of Age-Specific Insurer Perspective on Lifetime Cost Effectiveness of Colorectal Cancer Screening.Am J Gastroenterol. 2018 Dec;113(12):1754-1756. doi: 10.1038/s41395-018-0386-z. Epub 2018 Oct 29. Am J Gastroenterol. 2018. PMID: 30374119 Free PMC article.
-
Cost-Effectiveness of Colorectal Cancer Genetic Testing.Int J Environ Res Public Health. 2021 Aug 6;18(16):8330. doi: 10.3390/ijerph18168330. Int J Environ Res Public Health. 2021. PMID: 34444091 Free PMC article.
-
Effectiveness of Colorectal Cancer (CRC) Screening on All-Cause and CRC-Specific Mortality Reduction: A Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Mar 24;15(7):1948. doi: 10.3390/cancers15071948. Cancers (Basel). 2023. PMID: 37046609 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

