O-linked mucin-type glycosylation in breast cancer
- PMID: 29903935
- PMCID: PMC6103458
- DOI: 10.1042/BST20170483
O-linked mucin-type glycosylation in breast cancer
Abstract
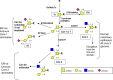
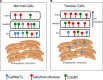
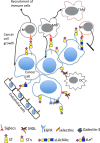
Changes in mucin-type O-linked glycosylation are seen in over 90% of breast cancers where increased sialylation is often observed and a change from branched glycans to linear glycans is often seen. There are many mechanisms involved including increased/altered expression of glycosyltransferases and relocalisation to the endoplasmic reticulum of the enzymes responsible for the addition of the first sugar, N-acetyl-d-galactosamine. It is now becoming clear that these changes can contribute to tumour growth and progression by modulating the micro-environment through glycan-sensing lectins expressed on immune cells, by modulating interactions with tumour surface receptors and by binding to selectins. The understanding of how changes in mucin-type O-linked glycosylation influence tumour growth and progression reveals new potential targets for therapeutic intervention in the treatment of breast cancer.
Keywords: O-linked mucin-type glycosylation; breast cancer; glycosyltransferases; immunotherapy; lectins.
© 2018 The Author(s).
Conflict of interest statement
J.M.B. is a consultant for Palleon Pharmaceuticals.
Figures
Similar articles
-
A transfected sialyltransferase that is elevated in breast cancer and localizes to the medial/trans-Golgi apparatus inhibits the development of core-2-based O-glycans.J Cell Biol. 1997 Jun 16;137(6):1229-41. doi: 10.1083/jcb.137.6.1229. J Cell Biol. 1997. PMID: 9182658 Free PMC article.
-
Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells.Eur J Biochem. 1995 Oct 15;233(2):607-17. doi: 10.1111/j.1432-1033.1995.607_2.x. Eur J Biochem. 1995. PMID: 7588808
-
The Close Relationship between the Golgi Trafficking Machinery and Protein Glycosylation.Cells. 2020 Dec 10;9(12):2652. doi: 10.3390/cells9122652. Cells. 2020. PMID: 33321764 Free PMC article. Review.
-
O-linked mucin-type glycosylation regulates the transcriptional programme downstream of EGFR.Glycobiology. 2021 Apr 1;31(3):200-210. doi: 10.1093/glycob/cwaa075. Glycobiology. 2021. PMID: 32776095
-
Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions.EMBO Rep. 2006 Jun;7(6):599-604. doi: 10.1038/sj.embor.7400705. EMBO Rep. 2006. PMID: 16741504 Free PMC article. Review.
Cited by
-
Rapid building block-economic synthesis of long, multi-O-GalNAcylated MUC5AC tandem repeat peptides.Chem Sci. 2023 Dec 18;15(4):1297-1305. doi: 10.1039/d3sc05006h. eCollection 2024 Jan 24. Chem Sci. 2023. PMID: 38274058 Free PMC article.
-
Measuring the multifaceted roles of mucin-domain glycoproteins in cancer.Adv Cancer Res. 2023;157:83-121. doi: 10.1016/bs.acr.2022.09.001. Epub 2022 Oct 8. Adv Cancer Res. 2023. PMID: 36725114 Free PMC article. Review.
-
A high heterozygosity genome assembly of Aedes albopictus enables the discovery of the association of PGANT3 with blood-feeding behavior.BMC Genomics. 2024 Apr 3;25(1):336. doi: 10.1186/s12864-024-10133-4. BMC Genomics. 2024. PMID: 38570743 Free PMC article.
-
GlycoEnzOnto: a GlycoEnzyme pathway and molecular function ontology.Bioinformatics. 2022 Dec 13;38(24):5413-5420. doi: 10.1093/bioinformatics/btac704. Bioinformatics. 2022. PMID: 36282863 Free PMC article.
-
CAR Based Immunotherapy of Solid Tumours-A Clinically Based Review of Target Antigens.Biology (Basel). 2023 Feb 10;12(2):287. doi: 10.3390/biology12020287. Biology (Basel). 2023. PMID: 36829563 Free PMC article. Review.
References
-
- Haltiwanger R.S., Wells L., Freeze H.H. and Stanley P. (2015–2017) Other Classes of Eukaryotic Glycans In Essentials of Glycobiology, 3rd edn (Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M. et al., eds), Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY) Chapter 13
-
- Stanley P., Taniguchi N. and Aebi M. (2015–2017) N-Glycans In Essentials of Glycobiology, 3rd edn (Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M. et al., eds), Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), Chapter 9
-
- Zachara N., Akimoto Y. and Hart G.W. (2015–2017) The O-GlcNAc modification In Essentials of Glycobiology, 3rd edn (Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M. et al., eds), Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), Chapter 19
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

