Autophagy Benefits the Replication of Egg Drop Syndrome Virus in Duck Embryo Fibroblasts
- PMID: 29896171
- PMCID: PMC5986908
- DOI: 10.3389/fmicb.2018.01091
Autophagy Benefits the Replication of Egg Drop Syndrome Virus in Duck Embryo Fibroblasts
Abstract
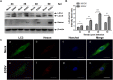
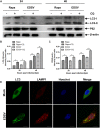
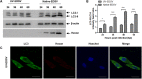
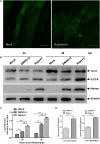
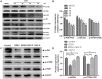
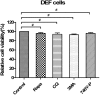
Egg drop syndrome virus (EDSV) is an economically important pathogen with a broad host range, and it causes disease that leads to markedly decreased egg production. Although EDSV is known to induce apoptosis in duck embryo fibroblasts (DEFs), the interaction between EDSV and its host needs to be further researched. Here, we provide the first evidence that EDSV infection triggers autophagy in DEFs through increases in autophagosome-like double-membrane vesicles, the conversion of LC3-I to LC3-II, and LC3 colocalization with viral hexon proteins. Conversely, P62/SQSTM1 degradation, LC3-II turnover, and colocalization of LAMP and LC3 confirmed that EDSV infection triggers complete autophagy. Furthermore, we demonstrated that inhibition of autophagy by chloroquine (CQ) and 3-methyladenine (3MA) or RNA interference targeting ATG-7 decreased the yield of EDSV progeny. In contrast, induction of autophagy by rapamycin increased the EDSV progeny yield. In addition, we preliminarily demonstrated that the class I phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway contributes to autophagic induction following EDSV infection. Altogether, these finding lead us to conclude that EDSV infection induces autophagy, which benefits its own replication in host cells. These findings provide novel insights into EDSV-host interactions.
Keywords: DEF cells; autophagic flux; autophagy; egg drop syndrome disease virus; virus replication.
Figures




Similar articles
-
Egg drop syndrome virus enters duck embryonic fibroblast cells via clathrin-mediated endocytosis.Virus Res. 2015 Dec 2;210:69-76. doi: 10.1016/j.virusres.2015.07.014. Epub 2015 Jul 19. Virus Res. 2015. PMID: 26200954
-
The role of hexon in egg drop syndrome virus (EDSV) inducing apoptosis in duck embryo fibroblast cells.Res Vet Sci. 2017 Oct;114:395-400. doi: 10.1016/j.rvsc.2017.07.015. Epub 2017 Jul 17. Res Vet Sci. 2017. PMID: 28743080
-
RNA-Seq analysis of duck embryo fibroblast cell gene expression during the early stage of egg drop syndrome virus infection.Poult Sci. 2019 Jan 1;98(1):404-412. doi: 10.3382/ps/pey318. Poult Sci. 2019. PMID: 30690613
-
Muscovy duck reovirus σNS protein triggers autophagy enhancing virus replication.Virol J. 2017 Mar 14;14(1):53. doi: 10.1186/s12985-017-0722-8. Virol J. 2017. PMID: 28288679 Free PMC article.
-
Autophagy activated by duck enteritis virus infection positively affects its replication.J Gen Virol. 2017 Mar;98(3):486-495. doi: 10.1099/jgv.0.000696. Epub 2017 Mar 20. J Gen Virol. 2017. PMID: 28008822
Cited by
-
The roles and mechanisms of endoplasmic reticulum stress-mediated autophagy in animal viral infections.Vet Res. 2024 Sep 3;55(1):107. doi: 10.1186/s13567-024-01360-4. Vet Res. 2024. PMID: 39227990 Free PMC article. Review.
-
Autophagy in farm animals: current knowledge and future challenges.Autophagy. 2021 Aug;17(8):1809-1827. doi: 10.1080/15548627.2020.1798064. Epub 2020 Jul 30. Autophagy. 2021. PMID: 32686564 Free PMC article. Review.
-
Downregulation of microRNA-30a-5p contributes to the replication of duck enteritis virus by regulating Beclin-1-mediated autophagy.Virol J. 2019 Nov 26;16(1):144. doi: 10.1186/s12985-019-1250-5. Virol J. 2019. PMID: 31771604 Free PMC article.
-
Critical Role of Viral Protein Hexon in Hypervirulent Fowl Adenovirus Serotype-4-Induced Autophagy by Interaction with BAG3 and Promotion of Viral Replication in LMH Cells.J Virol. 2023 Jun 29;97(6):e0028423. doi: 10.1128/jvi.00284-23. Epub 2023 May 31. J Virol. 2023. PMID: 37255472 Free PMC article.
-
The Multi-Faceted Role of Autophagy During Animal Virus Infection.Front Cell Infect Microbiol. 2022 Mar 25;12:858953. doi: 10.3389/fcimb.2022.858953. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402295 Free PMC article. Review.
References
-
- Bidin Z., Lojkic I., Mikec M., Pokric B. (2007). Naturally occurring egg drop syndrome infection in turkeys. Acta Vet. Brno 76 415–421. 10.2754/avb200776030415 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

