CDK-11-Cyclin L is required for gametogenesis and fertility in C. elegans
- PMID: 29886128
- PMCID: PMC8919330
- DOI: 10.1016/j.ydbio.2018.06.006
CDK-11-Cyclin L is required for gametogenesis and fertility in C. elegans
Abstract
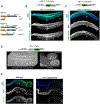
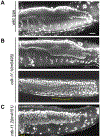
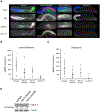
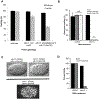
CDK11, a member of the cyclin-dependent kinase family, has been implicated in a diverse array of functions including transcription, RNA processing, sister chromatid cohesion, spindle assembly, centriole duplication and apoptosis. Despite its involvement in many essential functions, little is known about the requirements for CDK11 and its partner Cyclin L in a developing multicellular organism. Here we investigate the function of CDK11 and Cyclin L during development of the nematode Caenorhabditis elegans. Worms express two CDK11 proteins encoded by distinct loci: CDK-11.1 is essential for normal male and female fertility and is broadly expressed in the nuclei of somatic and germ line cells, while CDK-11.2 is nonessential and is enriched in hermaphrodite germ line nuclei beginning in mid pachytene. Hermaphrodites lacking CDK-11.1 develop normally but possess fewer mature sperm and oocytes and do not fully activate the RAS-ERK pathway that is required for oocyte production in response to environmental cues. Most of the sperm and eggs that are produced in cdk-11.1 null animals appear to complete development normally but fail to engage in sperm-oocyte signaling suggesting that CDK-11.1 is needed at multiple points in gametogenesis. Finally, we find that CDK-11.1 and CDK-11.2 function redundantly during embryonic and postembryonic development and likely do so in association with Cyclin L. Our results thus define multiple requirements for CDK-11-Cyclin L during animal development.
Keywords: C. elegans; CDK11; Cyclin L; Gametogenesis; RAS-ERK.
Published by Elsevier Inc.
Figures


Similar articles
-
Regulation of postembryonic G(1) cell cycle progression in Caenorhabditis elegans by a cyclin D/CDK-like complex.Development. 1999 Nov;126(21):4849-60. doi: 10.1242/dev.126.21.4849. Development. 1999. PMID: 10518501
-
The TRIM-NHL protein LIN-41 and the OMA RNA-binding proteins antagonistically control the prophase-to-metaphase transition and growth of Caenorhabditis elegans oocytes.Genetics. 2014 Dec;198(4):1535-58. doi: 10.1534/genetics.114.168831. Epub 2014 Sep 26. Genetics. 2014. PMID: 25261698 Free PMC article.
-
The Caenorhabditis elegans homologue of deleted in azoospermia is involved in the sperm/oocyte switch.Mol Biol Cell. 2006 Jul;17(7):3147-55. doi: 10.1091/mbc.e05-11-1067. Epub 2006 Apr 26. Mol Biol Cell. 2006. PMID: 16641369 Free PMC article.
-
Roles of CDK/Cyclin complexes in transcription and pre-mRNA splicing: Cyclins L and CDK11 at the cross-roads of cell cycle and regulation of gene expression.Semin Cell Dev Biol. 2020 Nov;107:36-45. doi: 10.1016/j.semcdb.2020.04.016. Epub 2020 May 20. Semin Cell Dev Biol. 2020. PMID: 32446654 Review.
-
The Emerging Picture of CDK11: Genetic, Functional and Medicinal Aspects.Curr Med Chem. 2018;25(8):880-888. doi: 10.2174/0929867324666170815102036. Curr Med Chem. 2018. PMID: 28814241 Review.
Cited by
-
Mitochondrial ribosomal small subunit (MRPS) MRPS23 protein-protein interaction reveals phosphorylation by CDK11-p58 affecting cell proliferation and knockdown of MRPS23 sensitizes breast cancer cells to CDK1 inhibitors.Mol Biol Rep. 2022 Oct;49(10):9521-9534. doi: 10.1007/s11033-022-07842-y. Epub 2022 Aug 13. Mol Biol Rep. 2022. PMID: 35962848
-
CDC37L1 acts as a suppressor of migration and proliferation in gastric cancer by down-regulating CDK6.J Cancer. 2021 Mar 31;12(11):3145-3153. doi: 10.7150/jca.56097. eCollection 2021. J Cancer. 2021. PMID: 33976724 Free PMC article.
References
-
- Ariza ME, Broome-Powell M, Lahti JM, Kidd VJ, Nelson MA, 1999. Fas-induced apoptosis in human malignant melanoma cell lines is associated with the activation of thep34(cdc2)-related PITSLRE protein kinases. J. Biol. Chem 274, 28505–28513. - PubMed
-
- Berke JD, Sgambato V, Zhu PP, Lavoie B, Vincent M, Krause M, Hyman SE, 2001. Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron 32, 277–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

