Immune Modulation by Human Secreted RNases at the Extracellular Space
- PMID: 29867984
- PMCID: PMC5964141
- DOI: 10.3389/fimmu.2018.01012
Immune Modulation by Human Secreted RNases at the Extracellular Space
Abstract
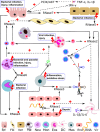
The ribonuclease A superfamily is a vertebrate-specific family of proteins that encompasses eight functional members in humans. The proteins are secreted by diverse innate immune cells, from blood cells to epithelial cells and their levels in our body fluids correlate with infection and inflammation processes. Recent studies ascribe a prominent role to secretory RNases in the extracellular space. Extracellular RNases endowed with immuno-modulatory and antimicrobial properties can participate in a wide variety of host defense tasks, from performing cellular housekeeping to maintaining body fluid sterility. Their expression and secretion are induced in response to a variety of injury stimuli. The secreted proteins can target damaged cells and facilitate their removal from the focus of infection or inflammation. Following tissue damage, RNases can participate in clearing RNA from cellular debris or work as signaling molecules to regulate the host response and contribute to tissue remodeling and repair. We provide here an overall perspective on the current knowledge of human RNases' biological properties and their role in health and disease. The review also includes a brief description of other vertebrate family members and unrelated extracellular RNases that share common mechanisms of action. A better knowledge of RNase mechanism of actions and an understanding of their physiological roles should facilitate the development of novel therapeutics.
Keywords: RNA; extracellular; infection; inflammation; innate immunity; ribonucleases.
Figures
Similar articles
-
Exploring the mechanisms of action of human secretory RNase 3 and RNase 7 against Candida albicans.Microbiologyopen. 2016 Oct;5(5):830-845. doi: 10.1002/mbo3.373. Epub 2016 Jun 8. Microbiologyopen. 2016. PMID: 27277554 Free PMC article.
-
The Ribonuclease A Superfamily in Humans: Canonical RNases as the Buttress of Innate Immunity.Int J Mol Sci. 2016 Aug 5;17(8):1278. doi: 10.3390/ijms17081278. Int J Mol Sci. 2016. PMID: 27527162 Free PMC article. Review.
-
A Review of Ribonuclease 7's Structure, Regulation, and Contributions to Host Defense.Int J Mol Sci. 2016 Mar 22;17(3):423. doi: 10.3390/ijms17030423. Int J Mol Sci. 2016. PMID: 27011175 Free PMC article. Review.
-
The RNase a superfamily: generation of diversity and innate host defense.Mol Divers. 2006 Nov;10(4):585-97. doi: 10.1007/s11030-006-9028-2. Mol Divers. 2006. PMID: 16969722 Review.
-
Antimicrobial RNases in cutaneous defense.J Innate Immun. 2012;4(3):241-7. doi: 10.1159/000335029. Epub 2012 Feb 10. J Innate Immun. 2012. PMID: 22327069 Free PMC article. Review.
Cited by
-
Efficacy and pharmacodynamic effect of anti-CD73 and anti-PD-L1 monoclonal antibodies in combination with cytotoxic therapy: observations from mouse tumor models.Cancer Biol Ther. 2024 Dec 31;25(1):2296048. doi: 10.1080/15384047.2023.2296048. Epub 2024 Jan 11. Cancer Biol Ther. 2024. PMID: 38206570 Free PMC article.
-
Exploring the RNase A scaffold to combine catalytic and antimicrobial activities. Structural characterization of RNase 3/1 chimeras.Front Mol Biosci. 2022 Sep 14;9:964717. doi: 10.3389/fmolb.2022.964717. eCollection 2022. Front Mol Biosci. 2022. PMID: 36188223 Free PMC article.
-
Immune Sensing Mechanisms that Discriminate Self from Altered Self and Foreign Nucleic Acids.Immunity. 2020 Jul 14;53(1):54-77. doi: 10.1016/j.immuni.2020.06.014. Immunity. 2020. PMID: 32668228 Free PMC article. Review.
-
A novel ligand-receptor relationship between families of ribonucleases and receptor tyrosine kinases.J Biomed Sci. 2018 Nov 19;25(1):83. doi: 10.1186/s12929-018-0484-7. J Biomed Sci. 2018. PMID: 30449278 Free PMC article. Review.
-
Delivery of mRNA Therapeutics for the Treatment of Hepatic Diseases.Mol Ther. 2019 Apr 10;27(4):794-802. doi: 10.1016/j.ymthe.2018.12.012. Epub 2018 Dec 22. Mol Ther. 2019. PMID: 30655211 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

