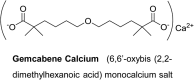
Gemcabene, a First-in-Class Hypolipidemic Small Molecule in Clinical Development, Attenuates Osteoarthritis and Pain in Animal Models of Arthritis and Pain
- PMID: 29867478
- PMCID: PMC5958179
- DOI: 10.3389/fphar.2018.00471
Gemcabene, a First-in-Class Hypolipidemic Small Molecule in Clinical Development, Attenuates Osteoarthritis and Pain in Animal Models of Arthritis and Pain
Abstract
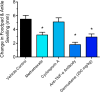
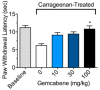
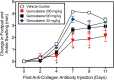
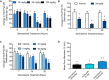
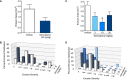
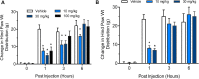
Our clinical studies have demonstrated that gemcabene, a small molecule in late-stage clinical development, lowers pro-inflammatory acute-phase protein, C-reactive protein (CRP). This observation was further confirmed in a cell-based study showing inhibition of cytokine-induced CRP production. Based on these observations, in the present study, we tested the hypothesis that gemcabene may possess anti-inflammatory activities in animal models of inflammatory disease. Efficacy of gemcabene was investigated in rat models of carrageenan-induced thermal hyperalgesia (CITH), monosodium iodoacetate (MIA)-induced osteoarthritis (OA), and IL-6/IL-6sR-induced inflammation. We also evaluated efficacy of gemcabene in collagen antibody-induced joint swelling and arthritis in BALB/c mice. In CITH rat model, gemcabene administration attenuated paw withdrawal latency (60% at 30 mg/kg/d and 97% at 100 mg/kg/d) and showed improvement in joint swelling (-50% at 30 mg/kg/d) in MIA model of OA. These findings were further corroborated by IL-6/IL-6sR knee injection model in rat, showing 63 and 71% reduction in hind paw weight distribution at 10 and 30 mg/kg/d doses, respectively. In mouse model of monoclonal antibody-induced arthritis, a dose-dependent attenuation of joint swelling was observed. These results demonstrate that the anti-inflammatory activity of gemcabene previously observed in cell-based and in clinical studies also occurred in animal models of inflammation-induced arthritis and hyperalgesia. Thus, in addition to hypolipidemic efficacy, the anti-inflammatory activity of gemcabene may have additional benefits to patients with elevated vascular inflammation.
Keywords: CRP; IL-1β; IL6; NF-kB; gemcabene; hyperalgesia; inflammation; osteoarthritis.
Figures
Similar articles
-
A Systematic Review of the Randomized Controlled Trials of Gemcabene and Its Therapeutic Uses.Cureus. 2023 Mar 28;15(3):e36811. doi: 10.7759/cureus.36811. eCollection 2023 Mar. Cureus. 2023. PMID: 37123792 Free PMC article. Review.
-
Gemcabene, a first-in-class lipid-lowering agent in late-stage development, down-regulates acute-phase C-reactive protein via C/EBP-δ-mediated transcriptional mechanism.Mol Cell Biochem. 2018 Dec;449(1-2):167-183. doi: 10.1007/s11010-018-3353-5. Epub 2018 Apr 11. Mol Cell Biochem. 2018. PMID: 29644527 Free PMC article.
-
Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats.BMC Complement Altern Med. 2019 Nov 21;19(1):325. doi: 10.1186/s12906-019-2746-7. BMC Complement Altern Med. 2019. PMID: 31752825 Free PMC article.
-
Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice--potential role of TRPA1 in osteoarthritis.Osteoarthritis Cartilage. 2015 Nov;23(11):2017-26. doi: 10.1016/j.joca.2015.09.008. Osteoarthritis Cartilage. 2015. PMID: 26521748
-
Model-based development of gemcabene, a new lipid-altering agent.AAPS J. 2005 Oct 7;7(3):E513-22. doi: 10.1208/aapsj070352. AAPS J. 2005. PMID: 16353929 Free PMC article. Review.
Cited by
-
Burn Ointment Promotes Cutaneous Wound Healing by Modulating the PI3K/AKT/mTOR Signaling Pathway.Front Pharmacol. 2021 Mar 8;12:631102. doi: 10.3389/fphar.2021.631102. eCollection 2021. Front Pharmacol. 2021. PMID: 33762951 Free PMC article.
-
Biological strategies for osteoarthritis: from early diagnosis to treatment.Int Orthop. 2021 Feb;45(2):335-344. doi: 10.1007/s00264-020-04838-w. Epub 2020 Oct 19. Int Orthop. 2021. PMID: 33078204 Review.
-
A Systematic Review of the Randomized Controlled Trials of Gemcabene and Its Therapeutic Uses.Cureus. 2023 Mar 28;15(3):e36811. doi: 10.7759/cureus.36811. eCollection 2023 Mar. Cureus. 2023. PMID: 37123792 Free PMC article. Review.
References
-
- Armitage J., Bowman L., Wallendszus K., Bulbulia R., Rahimi K., Haynes R., et al. (2010). Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. 376 1658–1669. 10.1016/S0140-6736(10)60310-8 - DOI - PMC - PubMed
-
- Arruda J. L., Sweitzer S., Rutkowski M. D., DeLeo J. A. (2000). Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. 879 216–225. - PubMed
-
- Ballantyne C. M., Davidson M. H., Macdougall D. E., Bays H. E., Dicarlo L. A., Rosenberg N. L., et al. (2013). Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. 62 1154–1162. 10.1016/j.jacc.2013.05.050 - DOI - PubMed
-
- Bendele A., McComb J., Gould T., McAbee T., Sennello G., Chlipala E., et al. (1999). Animal models of arthritis: relevance to human disease. 27 134–142. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

