Some Biological Consequences of the Inhibition of Na,K-ATPase by Translationally Controlled Tumor Protein (TCTP)
- PMID: 29867020
- PMCID: PMC6032315
- DOI: 10.3390/ijms19061657
Some Biological Consequences of the Inhibition of Na,K-ATPase by Translationally Controlled Tumor Protein (TCTP)
Abstract
Na,K-ATPase is an ionic pump that regulates the osmotic equilibrium and membrane potential of cells and also functions as a signal transducer. The interaction of Na,K-ATPase with translationally controlled tumor protein (TCTP) results, among others, in the inhibition of the former's pump activity and in the initiation of manifold biological and pathological phenomena. These phenomena include hypertension and cataract development in TCTP-overexpressing transgenic mice, as well as the induction of tumorigenesis signaling pathways and the activation of Src that ultimately leads to cell proliferation and migration. This review attempts to collate the biological effects of Na,K-ATPase and TCTP interaction and suggests that this interaction has the potential to serve as a possible therapeutic target for selected diseases.
Keywords: Na,K-ATPase; autophagy; hypertension; translationally controlled tumor protein; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
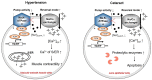
 , Na+;
, Na+;  Ca2+;
Ca2+;  , proteolytic enzymes).
, proteolytic enzymes).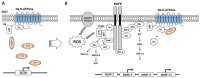
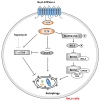
Similar articles
-
Transgenic overexpression of translationally controlled tumor protein induces systemic hypertension via repression of Na+,K+-ATPase.J Mol Cell Cardiol. 2008 Jan;44(1):151-9. doi: 10.1016/j.yjmcc.2007.09.017. Epub 2007 Oct 6. J Mol Cell Cardiol. 2008. PMID: 17976639
-
Over-expression of translationally controlled tumor protein in lens epithelial cells seems to be associated with cataract development.Transgenic Res. 2009 Dec;18(6):953-60. doi: 10.1007/s11248-009-9283-y. Epub 2009 May 29. Transgenic Res. 2009. PMID: 19479337
-
Inhibition of Na,K-ATPase-suppressive activity of translationally controlled tumor protein by sorting nexin 6.FEBS Lett. 2006 Jun 12;580(14):3558-64. doi: 10.1016/j.febslet.2006.05.042. Epub 2006 May 24. FEBS Lett. 2006. PMID: 16730713
-
Role of Translationally Controlled Tumor Protein (TCTP) in the Development of Hypertension and Related Diseases in Mouse Models.Biomedicines. 2022 Oct 27;10(11):2722. doi: 10.3390/biomedicines10112722. Biomedicines. 2022. PMID: 36359240 Free PMC article. Review.
-
Potassium, Na+-K+ pump inhibitor and low-renin hypertension.Clin Invest Med. 1987 Nov;10(6):547-54. Clin Invest Med. 1987. PMID: 2831002 Review.
Cited by
-
The multifaceted potential of TPT1 as biomarker and therapeutic target.Heliyon. 2024 Oct 1;10(19):e38819. doi: 10.1016/j.heliyon.2024.e38819. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39397949 Free PMC article. Review.
-
Tctp, a unique Ing5-binding partner, inhibits the chromatin binding of Enok in Drosophila.Proc Natl Acad Sci U S A. 2023 Apr 11;120(15):e2218361120. doi: 10.1073/pnas.2218361120. Epub 2023 Apr 4. Proc Natl Acad Sci U S A. 2023. PMID: 37014852 Free PMC article.
-
Regulation of Autophagy Is a Novel Tumorigenesis-Related Activity of Multifunctional Translationally Controlled Tumor Protein.Cells. 2020 Jan 20;9(1):257. doi: 10.3390/cells9010257. Cells. 2020. PMID: 31968668 Free PMC article. Review.
-
In Vitro Study of the Multimodal Effect of Na+/K+ ATPase Blocker Ouabain on the Tumor Microenvironment and Malignant Cells.Biomedicines. 2023 Aug 5;11(8):2205. doi: 10.3390/biomedicines11082205. Biomedicines. 2023. PMID: 37626702 Free PMC article.
-
Amiodarone exacerbates brain injuries after hypoxic-ischemic insult in mice.BMC Neurosci. 2019 Dec 21;20(1):62. doi: 10.1186/s12868-019-0544-2. BMC Neurosci. 2019. PMID: 31864286 Free PMC article.
References
-
- Friedrich B., Matskevich I., Lang F. Cell volume regulatory mechanisms. Contrib. Nephrol. 2006;152:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

