Efficacy and safety of sofosbuvir plus ribavirin for Korean patients with hepatitis C virus genotype 2 infection: A retrospective multi-institutional study
- PMID: 29865774
- PMCID: PMC6166109
- DOI: 10.3350/cmh.2017.0070
Efficacy and safety of sofosbuvir plus ribavirin for Korean patients with hepatitis C virus genotype 2 infection: A retrospective multi-institutional study
Abstract
Background/aims: Sofosbuvir plus ribavirin is a standard treatment for patients infected with chronic hepatitis C virus (HCV) genotype 2 in Korea. The purpose of this study was to examine the efficacy and safety of this treatment in Korean patients with chronic HCV genotype 2 infection.
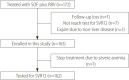
Methods: We retrospectively analyzed clinical data of patients treated with sofosbuvir plus ribavirin for chronic HCV genotype 2 from May 2016 to December 2017 at eight hospitals located in the Daejeon-Chungcheong area.
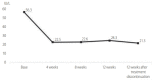
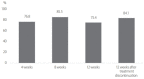
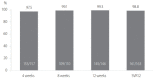
Results: A total of 172 patients were treated with sofosbuvir plus ribavirin. Of them, 163 patients completed the treatment, and 162 patients were tested for sustained virologic response 12 weeks after treatment discontinuation (SVR12). Mean age was 59.6±12.3 years (27-96), and 105 (64.4%) patients were female. Of the total patients, 49 (30.1%) were diagnosed with cirrhosis, and 31 of them were treated for 16 weeks. Sofosbuvir plus ribavirin was the first-line treatment for 144 (88.3%) patients. Eleven (6.7%) patients were intolerant to previous interferon-based treatment. Eight (5.0%) patients relapsed after interferon-based treatment. HCV RNA non-detection rate at 4, 8, and 12 weeks was 97.5%, 99.1%, and 99.3%, respectively, and SVR12 was 98.8% (161/163). During treatment, 18 (11.0%) patients had to reduce their administrated dose of ribavirin because of anemia. One patient stopped the treatment because of severe anemia. Other adverse events, including dizziness, indigestion, and headache, were found in 26 (16.0%) patients.
Conclusion: A 12-16 week treatment with sofosbuvir plus ribavirin is remarkably effective and well tolerated in Korean patients with chronic HCV genotype 2 infection.
Keywords: Hepatitis C virus; Korea; Ribavirin; Sofosbuvir.
Conflict of interest statement
SB Kim, SH Lee, BS Lee, JW Jang receives grants from Gilead Sciences. The other authors declare no conflicts of interest.
Figures

Comment in
-
Does the old-fashioned sofosbuvir plus ribavirin treatment in genotype 2 chronic hepatitis C patients still works for Koreans?Clin Mol Hepatol. 2018 Sep;24(3):294-296. doi: 10.3350/cmh.2018.1009. Epub 2018 Sep 11. Clin Mol Hepatol. 2018. PMID: 30200750 Free PMC article. No abstract available.
Similar articles
-
A phase 3b study of sofosbuvir plus ribavirin in treatment-naive and treatment-experienced Korean patients chronically infected with genotype 2 hepatitis C virus.J Viral Hepat. 2016 May;23(5):358-65. doi: 10.1111/jvh.12499. Epub 2016 Feb 10. J Viral Hepat. 2016. PMID: 26864153 Clinical Trial.
-
Sofosbuvir Plus Velpatasvir Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial.Ann Intern Med. 2015 Dec 1;163(11):809-17. doi: 10.7326/M15-1014. Epub 2015 Nov 10. Ann Intern Med. 2015. PMID: 26551263 Clinical Trial.
-
Sofosbuvir-based therapy for patients with chronic hepatitis C: Early experience of its efficacy and safety in Korea.Clin Mol Hepatol. 2015 Dec;21(4):358-64. doi: 10.3350/cmh.2015.21.4.358. Epub 2015 Dec 24. Clin Mol Hepatol. 2015. PMID: 26770924 Free PMC article.
-
Safety and efficacy of sofosbuvir plus velpatasvir with or without ribavirin for chronic hepatitis C virus infection: A systematic review and meta-analysis.J Infect Public Health. 2018 Mar-Apr;11(2):156-164. doi: 10.1016/j.jiph.2017.09.004. Epub 2017 Sep 29. J Infect Public Health. 2018. PMID: 28970099 Review.
-
Daclatasvir and Sofosbuvir Versus Sofosbuvir and Ribavirin in Patients with Chronic Hepatitis C Coinfected with HIV: A Matching-adjusted Indirect Comparison.Clin Ther. 2016 Feb;38(2):404-12. doi: 10.1016/j.clinthera.2015.12.017. Epub 2016 Feb 3. Clin Ther. 2016. PMID: 26839044 Review.
Cited by
-
Efficacy and Safety of Sofosbuvir and Ribavirin in an Italian Cohort of HCV Genotype 2 Elderly Cirrhotic Patients.Eurasian J Med. 2022 Jun;54(2):113-120. doi: 10.5152/eurasianjmed.2022.20421. Eurasian J Med. 2022. PMID: 35703517 Free PMC article.
-
Comparison of the Clinical Characteristics and Outcomes between Leprosy-Affected Persons in Sorokdo and the General Population Affected by Chronic Hepatitis C in Korea.Gut Liver. 2019 Sep 15;13(5):549-556. doi: 10.5009/gnl18432. Gut Liver. 2019. PMID: 30970433 Free PMC article.
-
Sofosbuvir-based therapies in genotype 2 hepatitis C virus cirrhosis: A real-life experience with focus on ribavirin dose.Pharmacol Res Perspect. 2021 Aug;9(4):e00811. doi: 10.1002/prp2.811. Pharmacol Res Perspect. 2021. PMID: 34152088 Free PMC article.
-
Real-Life Effectiveness and Safety of Glecaprevir/Pibrentasvir for Korean Patients with Chronic Hepatitis C at a Single Institution.Gut Liver. 2021 May 15;15(3):440-450. doi: 10.5009/gnl19393. Gut Liver. 2021. PMID: 32839365 Free PMC article.
-
Hepatocellular Carcinoma Risk According to Regimens for Eradication of Hepatitis C Virus; Interferon or Direct Acting Antivirals.Cancers (Basel). 2020 Nov 18;12(11):3414. doi: 10.3390/cancers12113414. Cancers (Basel). 2020. PMID: 33217965 Free PMC article.
References
-
- Kim DY, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013;33:586–594. - PubMed
-
- Kanda T, Imazeki F, Azemoto R, Yonemitsu Y, Mikami S, Kita K, et al. Response to peginterferon-alfa 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 2. Dig Dis Sci. 2011;56:3335–3342. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

