Synthetic vaccine particles for durable cytolytic T lymphocyte responses and anti-tumor immunotherapy
- PMID: 29856772
- PMCID: PMC5983463
- DOI: 10.1371/journal.pone.0197694
Synthetic vaccine particles for durable cytolytic T lymphocyte responses and anti-tumor immunotherapy
Abstract
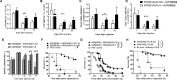
We previously reported that synthetic vaccine particles (SVP) encapsulating antigens and TLR agonists resulted in augmentation of immune responses with minimal production of systemic inflammatory cytokines. Here we evaluated two different polymer formulations of SVP-encapsulated antigens and tested their ability to induce cytolytic T lymphocytes (CTL) in combination with SVP-encapsulated adjuvants. One formulation led to efficient antigen processing and cross-presentation, rapid and sustained CTL activity, and expansion of CD8+ T cell effector memory cells locally and centrally, which persisted for at least 1-2 years after a single immunization. SVP therapeutic dosing resulted in suppression of tumor growth and a substantial delay in mortality in several syngeneic mouse cancer models. Treatment with checkpoint inhibitors and/or cytotoxic drugs, while suboptimal on their own, showed considerable synergy with SVP immunization. SVP encapsulation of endosomal TLR agonists provided superior CTL induction, therapeutic benefit and/or improved safety profile compared to free adjuvants. SVP vaccines encapsulating mutated HPV-16 E7 and E6/E7 recombinant proteins led to induction of broad CTL activity and strong inhibition of TC-1 tumor growth, even when administered therapeutically 13-14 days after tumor inoculation in animals bearing palpable tumors. A pilot study in non-human primates showed that SVP-encapsulated E7/E6 adjuvanted with SVP-encapsulated poly(I:C) led to robust induction of antigen-specific T and B cell responses.
Conflict of interest statement
All authors are employees and shareholders of Selecta Biosciences and SelectaRUS. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


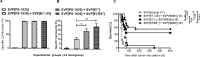





Similar articles
-
Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication.BMC Cancer. 2019 Jun 6;19(1):540. doi: 10.1186/s12885-019-5725-y. BMC Cancer. 2019. PMID: 31170937 Free PMC article.
-
Flagellin enhances tumor-specific CD8⁺ T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model.Vaccine. 2013 Aug 20;31(37):3879-87. doi: 10.1016/j.vaccine.2013.06.054. Epub 2013 Jul 2. Vaccine. 2013. PMID: 23831323
-
Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells.Arch Virol. 2018 Mar;163(3):587-597. doi: 10.1007/s00705-017-3647-z. Epub 2017 Nov 17. Arch Virol. 2018. PMID: 29149434
-
Understanding how combinatorial targeting of TLRs and TNFR family costimulatory members promote enhanced T cell responses.Expert Opin Biol Ther. 2018 Oct;18(10):1073-1083. doi: 10.1080/14712598.2018.1518422. Epub 2018 Sep 12. Expert Opin Biol Ther. 2018. PMID: 30169979 Review.
-
Development of cancer vaccines to activate cytotoxic T lymphocytes.Expert Opin Biol Ther. 2005 Apr;5(4):555-63. doi: 10.1517/14712598.5.4.555. Expert Opin Biol Ther. 2005. PMID: 15934833 Review.
Cited by
-
Advanced Nanomedicine for High-Risk HPV-Driven Head and Neck Cancer.Viruses. 2022 Dec 19;14(12):2824. doi: 10.3390/v14122824. Viruses. 2022. PMID: 36560828 Free PMC article. Review.
-
MuSyC dosing of adjuvanted cancer vaccines optimizes antitumor responses.Front Immunol. 2022 Aug 19;13:936129. doi: 10.3389/fimmu.2022.936129. eCollection 2022. Front Immunol. 2022. PMID: 36059502 Free PMC article.
-
The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy.Pharmaceutics. 2022 Jun 10;14(6):1228. doi: 10.3390/pharmaceutics14061228. Pharmaceutics. 2022. PMID: 35745800 Free PMC article. Review.
-
Tumor mutational burden presents limiting effects on predicting the efficacy of immune checkpoint inhibitors and prognostic assessment in adrenocortical carcinoma.BMC Endocr Disord. 2022 May 14;22(1):130. doi: 10.1186/s12902-022-01017-3. BMC Endocr Disord. 2022. PMID: 35568842 Free PMC article.
-
HPV16 E6-specific T cell response and HLA-A alleles are related to the prognosis of patients with cervical cancer.Infect Agent Cancer. 2021 Sep 16;16(1):61. doi: 10.1186/s13027-021-00395-y. Infect Agent Cancer. 2021. PMID: 34530896 Free PMC article.
References
-
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012; 62: 309–335. doi: 10.3322/caac.20132 - DOI - PMC - PubMed
-
- Sharma P, and Allison JP. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015; 161: 205–214. doi: 10.1016/j.cell.2015.03.030 - DOI - PMC - PubMed
-
- Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016; 13:273–290. doi: 10.1038/nrclinonc.2016.25 - DOI - PMC - PubMed
-
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. doi: 10.1056/NEJMoa1407222 - DOI - PMC - PubMed
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528. doi: 10.1016/S0140-6736(14)61403-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

