The cJUN NH2-terminal kinase (JNK) signaling pathway promotes genome stability and prevents tumor initiation
- PMID: 29856313
- PMCID: PMC5984035
- DOI: 10.7554/eLife.36389
The cJUN NH2-terminal kinase (JNK) signaling pathway promotes genome stability and prevents tumor initiation
Abstract
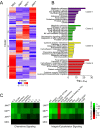
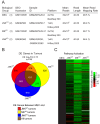
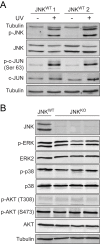
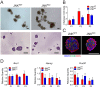
Breast cancer is the most commonly diagnosed malignancy in women. Analysis of breast cancer genomic DNA indicates frequent loss-of-function mutations in components of the cJUN NH2-terminal kinase (JNK) signaling pathway. Since JNK signaling can promote cell proliferation by activating the AP1 transcription factor, this apparent association of reduced JNK signaling with tumor development was unexpected. We examined the effect of JNK deficiency in the murine breast epithelium. Loss of JNK signaling caused genomic instability and the development of breast cancer. Moreover, JNK deficiency caused widespread early neoplasia and rapid tumor formation in a murine model of breast cancer. This tumor suppressive function was not mediated by a role of JNK in the growth of established tumors, but by a requirement of JNK to prevent tumor initiation. Together, these data identify JNK pathway defects as 'driver' mutations that promote genome instability and tumor initiation.
Keywords: JNK; breast cancer; cancer biology; cell biology; mouse; stress-activated MAPK.
© 2018, Girnius et al.
Conflict of interest statement
NG, YE, DG No competing interests declared, RD Reviewing editor, eLife
Figures

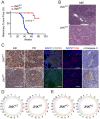
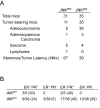
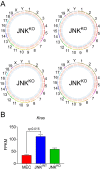
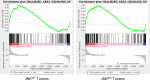
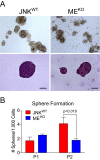
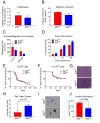
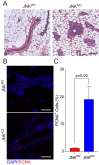
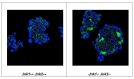
Similar articles
-
c-Jun N-terminal kinase promotes stem cell phenotype in triple-negative breast cancer through upregulation of Notch1 via activation of c-Jun.Oncogene. 2017 May 4;36(18):2599-2608. doi: 10.1038/onc.2016.417. Epub 2016 Dec 12. Oncogene. 2017. PMID: 27941886 Free PMC article.
-
Stress-activated kinase pathway alteration is a frequent event in bladder cancer.Urol Oncol. 2012 Jul-Aug;30(4):415-20. doi: 10.1016/j.urolonc.2010.03.002. Epub 2011 Dec 11. Urol Oncol. 2012. PMID: 22154358
-
Breast cancer cells proliferation is regulated by tyrosine phosphatase SHP1 through c-jun N-terminal kinase and cooperative induction of RFX-1 and AP-4 transcription factors.Mol Cancer Res. 2011 Aug;9(8):1112-25. doi: 10.1158/1541-7786.MCR-11-0097. Epub 2011 Jun 30. Mol Cancer Res. 2011. PMID: 21719561
-
The Jun N-terminal kinases signaling pathway plays a "seesaw" role in ovarian carcinoma: a molecular aspect.J Ovarian Res. 2019 Oct 21;12(1):99. doi: 10.1186/s13048-019-0573-6. J Ovarian Res. 2019. PMID: 31639019 Free PMC article. Review.
-
JNK Signaling in Stem Cell Self-Renewal and Differentiation.Int J Mol Sci. 2020 Apr 9;21(7):2613. doi: 10.3390/ijms21072613. Int J Mol Sci. 2020. PMID: 32283767 Free PMC article. Review.
Cited by
-
Therapeutic and pharmacological efficacy of plant-derived bioactive compounds in targeting breast cancer.Am J Transl Res. 2024 May 15;16(5):1499-1520. doi: 10.62347/NUZN4999. eCollection 2024. Am J Transl Res. 2024. PMID: 38883353 Free PMC article. Review.
-
Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis.EMBO Mol Med. 2018 Oct;10(10):e9003. doi: 10.15252/emmm.201809003. EMBO Mol Med. 2018. PMID: 30190333 Free PMC article.
-
ROS-mediated SRMS activation confers platinum resistance in ovarian cancer.Oncogene. 2023 May;42(20):1672-1684. doi: 10.1038/s41388-023-02679-6. Epub 2023 Apr 5. Oncogene. 2023. PMID: 37020040 Free PMC article.
-
JNK in Tumor Microenvironment: Present Findings and Challenges in Clinical Translation.Cancers (Basel). 2021 May 3;13(9):2196. doi: 10.3390/cancers13092196. Cancers (Basel). 2021. PMID: 34063627 Free PMC article. Review.
-
Aggresome formation promotes ASK1/JNK signaling activation and stemness maintenance in ovarian cancer.Nat Commun. 2024 Feb 13;15(1):1321. doi: 10.1038/s41467-024-45698-x. Nat Commun. 2024. PMID: 38351029 Free PMC article.
References
-
- Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, Ardlie KG, Auclair D, Bautista-Piña V, Duke F, Francis J, Jung J, Maffuz-Aziz A, Onofrio RC, Parkin M, Pho NH, Quintanar-Jurado V, Ramos AH, Rebollar-Vega R, Rodriguez-Cuevas S, Romero-Cordoba SL, Schumacher SE, Stransky N, Thompson KM, Uribe-Figueroa L, Baselga J, Beroukhim R, Polyak K, Sgroi DC, Richardson AL, Jimenez-Sanchez G, Lander ES, Gabriel SB, Garraway LA, Golub TR, Melendez-Zajgla J, Toker A, Getz G, Hidalgo-Miranda A, Meyerson M. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. - DOI - PMC - PubMed
-
- Berger AH, Brooks AN, Wu X, Shrestha Y, Chouinard C, Piccioni F, Bagul M, Kamburov A, Imielinski M, Hogstrom L, Zhu C, Yang X, Pantel S, Sakai R, Watson J, Kaplan N, Campbell JD, Singh S, Root DE, Narayan R, Natoli T, Lahr DL, Tirosh I, Tamayo P, Getz G, Wong B, Doench J, Subramanian A, Golub TR, Meyerson M, Boehm JS. High-throughput phenotyping of lung Cancer somatic mutations. Cancer Cell. 2016;30:214–228. doi: 10.1016/j.ccell.2016.06.022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

