Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms
- PMID: 29852857
- PMCID: PMC7040520
- DOI: 10.2174/0929867325666180601101859
Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms
Abstract
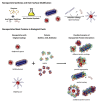
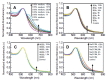
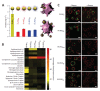
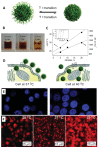
Within the last two decades, the field of nanomedicine has not developed as successfully as has widely been hoped for. The main reason for this is the immense complexity of the biological systems, including the physico-chemical properties of the biological fluids as well as the biochemistry and the physiology of living systems. The nanoparticles' physicochemical properties are also highly important. These differ profoundly from those of freshly synthesized particles when applied in biological/living systems as recent research in this field reveals. The physico-chemical properties of nanoparticles are predefined by their structural and functional design (core and coating material) and are highly affected by their interaction with the environment (temperature, pH, salt, proteins, cells). Since the coating material is the first part of the particle to come in contact with the environment, it does not only provide biocompatibility, but also defines the behavior (e.g. colloidal stability) and the fate (degradation, excretion, accumulation) of nanoparticles in the living systems. Hence, the coating matters, particularly for a nanoparticle system for biomedical applications, which has to fulfill its task in the complex environment of biological fluids, cells and organisms. In this review, we evaluate the performance of different coating materials for nanoparticles concerning their ability to provide colloidal stability in biological media and living systems.
Keywords: Nanoparticles; biological media; biopolymers; coating materials; colloidal stability; polymeric coatings; protein corona..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures

Similar articles
-
Recent Developments in the Design of Non-Biofouling Coatings for Nanoparticles and Surfaces.Int J Mol Sci. 2020 Feb 3;21(3):1007. doi: 10.3390/ijms21031007. Int J Mol Sci. 2020. PMID: 32028729 Free PMC article. Review.
-
Amphoteric natural starch-coated polymer nanoparticles with excellent protein corona-free and targeting properties.Nanoscale. 2020 Mar 14;12(10):5834-5847. doi: 10.1039/c9nr09405a. Epub 2020 Feb 18. Nanoscale. 2020. PMID: 32068222
-
Cellular Uptake of Upconversion Nanoparticles Based on Surface Polymer Coatings and Protein Corona.ACS Appl Mater Interfaces. 2024 Jul 17;16(28):35985-36001. doi: 10.1021/acsami.4c04148. Epub 2024 Jul 3. ACS Appl Mater Interfaces. 2024. PMID: 38958411
-
On the formation of protein corona on colloidal nanoparticles stabilized by depletant polymers.Mater Sci Eng C Mater Biol Appl. 2019 Dec;105:110080. doi: 10.1016/j.msec.2019.110080. Epub 2019 Aug 13. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31546390
-
Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System.Semin Immunol. 2017 Dec;34:52-60. doi: 10.1016/j.smim.2017.10.001. Epub 2017 Oct 21. Semin Immunol. 2017. PMID: 29066063 Review.
Cited by
-
Synthesis of metallic nanoparticles using biometabolites: mechanisms and applications.Biometals. 2024 Oct 8. doi: 10.1007/s10534-024-00642-w. Online ahead of print. Biometals. 2024. PMID: 39377881 Review.
-
Effect of Polygonatum sibiricum on biological toxicity of zinc oxide nanoparticles during respiratory exposure.RSC Adv. 2024 Oct 2;14(43):31360-31366. doi: 10.1039/d4ra03738c. eCollection 2024 Oct 1. RSC Adv. 2024. PMID: 39359342 Free PMC article.
-
Effect of mesenchymal stem cells and polyvinyl alcohol-coated selenium nanoparticles on rats with Alzheimer-like phenotypes.Iran J Basic Med Sci. 2024;27(10):1268-1275. doi: 10.22038/ijbms.2024.76242.16495. Iran J Basic Med Sci. 2024. PMID: 39229584 Free PMC article.
-
Ion carrier modulated MRI contrast.Chem Sci. 2024 Jul 18;15(34):13937-41. doi: 10.1039/d3sc03466f. Online ahead of print. Chem Sci. 2024. PMID: 39129769 Free PMC article.
-
Polydopamine Nanoparticles as Mimicking RPE Melanin for the Protection of Retinal Cells Against Blue Light-Induced Phototoxicity.Adv Sci (Weinh). 2024 Aug;11(29):e2400230. doi: 10.1002/advs.202400230. Epub 2024 May 30. Adv Sci (Weinh). 2024. PMID: 38816934 Free PMC article.
References
-
- Winau F., Westphal O., Winau R. Paul Ehrlich--in search of the magic bullet. Microbes Infect. 2004;6(8):786–789. - PubMed
-
- Abbott N.J., Romero I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today. 1996;2(3):106–113. - PubMed
-
- Brannon-Peppas L., Blanchette J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004;56(11):1649–1659. - PubMed
-
- Fabio S., Catherine D., Paolo C., Patrick C. Metallic Col-loid Nanotechnology, Applications in Diagnosis and Thera-peutics. Curr. Pharm. Des. 2005;11(16):2091–2105.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

