microRNAs and cardiac stem cells in heart development and disease
- PMID: 29852125
- PMCID: PMC6261710
- DOI: 10.1016/j.drudis.2018.05.032
microRNAs and cardiac stem cells in heart development and disease
Abstract
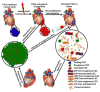
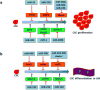
Cumulative evidence has proven that proliferation, differentiation and migration of cardiac stem cells (CSCs) dominate early heart development and contribute to the later occurrence of heart disease. Among other mechanisms, microRNAs work as the 'fine-tuning' to modulate the levels of target genes in a specific cell type. The distinct microRNA signatures in CSCs reveal the stages and functions of CSCs. The focus of this review is to summarize recent knowledge advances in CSC proliferation, differentiation and migration and to discuss how microRNAs regulate these processes during heart development and in heart disease. Better understanding of microRNA regulation on CSCs under different situations will enable the unveiling of the mechanisms of heart disease and open new avenues in the therapeutic potentials of microRNA modulation to treat heart disease.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest regarding the publication of this paper.
Figures

Similar articles
-
Up-Regulation of miRNA-21 Expression Promotes Migration and Proliferation of Sca-1+ Cardiac Stem Cells in Mice.Med Sci Monit. 2016 May 23;22:1724-32. doi: 10.12659/msm.895753. Med Sci Monit. 2016. PMID: 27210794 Free PMC article.
-
Endogenous cardiac stem cells.Prog Cardiovasc Dis. 2007 Jul-Aug;50(1):31-48. doi: 10.1016/j.pcad.2007.03.005. Prog Cardiovasc Dis. 2007. PMID: 17631436 Review.
-
Regulation of cardiac stem cells by microRNAs: State-of-the-art.Biomed Pharmacother. 2019 Dec;120:109447. doi: 10.1016/j.biopha.2019.109447. Epub 2019 Sep 30. Biomed Pharmacother. 2019. PMID: 31580971 Review.
-
MicroRNAs in stem cell function and regenerative therapy of the heart.Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):1739-46. doi: 10.1161/ATVBAHA.113.300138. Arterioscler Thromb Vasc Biol. 2013. PMID: 23864723 Review.
-
Small engine, big power: microRNAs as regulators of cardiac diseases and regeneration.Int J Mol Sci. 2014 Sep 9;15(9):15891-911. doi: 10.3390/ijms150915891. Int J Mol Sci. 2014. PMID: 25207600 Free PMC article. Review.
Cited by
-
Recent insights into the microRNA and long non-coding RNA-mediated regulation of stem cell populations.3 Biotech. 2022 Oct;12(10):270. doi: 10.1007/s13205-022-03343-8. Epub 2022 Sep 10. 3 Biotech. 2022. PMID: 36101546 Free PMC article. Review.
-
The Role of MicroRNA in the Myocarditis: a Small Actor for a Great Role.Curr Cardiol Rep. 2023 Jul;25(7):641-648. doi: 10.1007/s11886-023-01888-5. Epub 2023 Jun 3. Curr Cardiol Rep. 2023. PMID: 37269474 Free PMC article. Review.
-
Insights into role of microRNAs in cardiac development, cardiac diseases, and developing novel therapies.Iran J Basic Med Sci. 2020 Aug;23(8):961-969. doi: 10.22038/ijbms.2020.40974.10015. Iran J Basic Med Sci. 2020. PMID: 32952941 Free PMC article. Review.
-
Human platelet lysate combined with mesenchymal stem cells pretreated with platelet lysate improved cardiac function in rats with myocardial infarction.Sci Rep. 2024 Nov 12;14(1):27701. doi: 10.1038/s41598-024-79050-6. Sci Rep. 2024. PMID: 39533052 Free PMC article.
-
Mitochondrial MiRNA in Cardiovascular Function and Disease.Cells. 2019 Nov 21;8(12):1475. doi: 10.3390/cells8121475. Cells. 2019. PMID: 31766319 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

