Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D
- PMID: 29851137
- PMCID: PMC6138489
- DOI: 10.1111/bcp.13652
Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D
Abstract
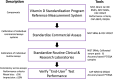
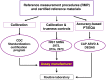
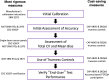
The First International Conference on Controversies in Vitamin D was held in Pisa, Italy, 14-16 June 2017. The meeting's purpose was to address controversies in vitamin D research, review the data available, to help resolve them, and suggest a research agenda to clarify areas of uncertainty. The serum 25-hydroxyvitamin D [25(OH)D] concentration [i.e. the sum of 25(OH)D3 and 25(OH)D2 ] remains the critical measurement for defining vitamin D status. Assay variation for 25(OH)D has contributed to the current chaos surrounding efforts to define hypovitaminosis D. An essential requirement to develop a consensus on vitamin D status is that measurement of 25(OH)D and, in the future, other potential vitamin D biomarkers [e.g. 1α,25(OH)2 D3 , 3-epi-25(OH)D, 24,25(OH)2 D3, vitamin D-binding protein, free/bioavailable 25(OH)D and parathyroid hormone] be standardized/harmonized, to allow pooling of research data. Vitamin D Standardization Program tools are described and recommended for standardizing 25(OH)D measurement in research. In the future, similar methodology, based on National Institute for Standards and Technology standard reference materials, must be developed for other candidate markers of vitamin D status. Failure to standardize/harmonize vitamin D metabolite measurements is destined to promulgate continued chaos. At this time, 25(OH)D values below 12 ng ml-1 (30 nmol l-1 ) should be considered to be associated with an increased risk of rickets/osteomalacia, whereas 25(OH)D concentrations between 20 ng ml-1 and 50 ng ml-1 (50-125 nmol l-1 ) appear to be safe and sufficient in the general population for skeletal health. In an effort to bridge knowledge gaps in defining hypovitaminosis D, an international study on rickets as a multifactorial disease is proposed.
Keywords: 25-hydroxyvitamin D; Fibroblast Growth Factor (FGF23); Parathyroid Hormone (PTH); Vitamin D; Vitamin D Standardization Program (VDSP); Vitamin D-binding protein (DBP).
© 2018 The British Pharmacological Society.
Figures
Comment in
-
Lack of vitamin D predicts impaired long-term immune response to COVID-19 vaccination.Endocrine. 2023 Dec;82(3):536-541. doi: 10.1007/s12020-023-03481-w. Epub 2023 Aug 17. Endocrine. 2023. PMID: 37592162 Free PMC article.
Similar articles
-
Vitamin D measurement standardization: The way out of the chaos.J Steroid Biochem Mol Biol. 2017 Oct;173:117-121. doi: 10.1016/j.jsbmb.2016.12.002. Epub 2016 Dec 12. J Steroid Biochem Mol Biol. 2017. PMID: 27979577 Review.
-
Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: a cross-sectional study.Eur J Pediatr. 2013 Dec;172(12):1607-17. doi: 10.1007/s00431-013-2119-z. Eur J Pediatr. 2013. PMID: 23959324
-
Assessment of serum total 25-hydroxyvitamin D assay commutability of Standard Reference Materials and College of American Pathologists Accuracy-Based Vitamin D (ABVD) Scheme and Vitamin D External Quality Assessment Scheme (DEQAS) materials: Vitamin D Standardization Program (VDSP) Commutability Study 2.Anal Bioanal Chem. 2021 Aug;413(20):5067-5084. doi: 10.1007/s00216-021-03470-w. Epub 2021 Jun 28. Anal Bioanal Chem. 2021. PMID: 34184102 Free PMC article.
-
Development and application of a LC-MS/MS assay for simultaneous analysis of 25-hydroxyvitamin-D and 3-epi-25-hydroxyvitamin-D metabolites in canine serum.J Steroid Biochem Mol Biol. 2020 May;199:105598. doi: 10.1016/j.jsbmb.2020.105598. Epub 2020 Jan 17. J Steroid Biochem Mol Biol. 2020. PMID: 31958632
-
Toward Clarity in Clinical Vitamin D Status Assessment: 25(OH)D Assay Standardization.Endocrinol Metab Clin North Am. 2017 Dec;46(4):885-899. doi: 10.1016/j.ecl.2017.07.012. Epub 2017 Sep 29. Endocrinol Metab Clin North Am. 2017. PMID: 29080641 Review.
Cited by
-
Effects of Vitamin D with Calcium and Associations of Mean 25-Hydroxyvitamin D Levels with 3-Year Change in Muscle Performance in Healthy Older Adults in the Boston STOP IT Trial.Calcif Tissue Int. 2022 Dec;111(6):580-586. doi: 10.1007/s00223-022-01024-5. Epub 2022 Sep 26. Calcif Tissue Int. 2022. PMID: 36161344
-
C3-epi-25(OH)D3 percentage, not level, may be a potential biomarker to reflect its pathological increase in multiple diseases: a cross-sectional case-control study.Sci Rep. 2023 Dec 27;13(1):23004. doi: 10.1038/s41598-023-50524-3. Sci Rep. 2023. PMID: 38155294 Free PMC article.
-
The Effect of Vitamin D Supplementation on its Metabolism and the Vitamin D Metabolite Ratio.Nutrients. 2019 Oct 21;11(10):2539. doi: 10.3390/nu11102539. Nutrients. 2019. PMID: 31640241 Free PMC article. Clinical Trial.
-
Vitamin D Status Increases During Pregnancy and in Response to Vitamin D Supplementation in Rural Gambian Women.J Nutr. 2020 Mar 1;150(3):492-504. doi: 10.1093/jn/nxz290. J Nutr. 2020. PMID: 31834380 Free PMC article.
-
Reconsidering vitamin D optimal values based on parathyroid hormone levels in a North Algerian cohort: stratification by gender and season.Arch Osteoporos. 2022 Jul 27;17(1):100. doi: 10.1007/s11657-022-01137-2. Arch Osteoporos. 2022. PMID: 35895238
References
-
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol 2017; 13: 466–479. - PubMed
-
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington, DC: National Academies Press. 2011. pp. 1115. - PubMed
-
- Holick MF, Binkley N, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. - PubMed
-
- Scientific Advisory Committee on Nutrition (SACN) . SACN vitamin D and health report. Public Health England, 2016.
-
- Binkley N, Dawson‐Hughes B, Durazo‐Arvizu R, Thamm M, Tian L, Merkel JM, et al Vitamin D measurement standardization: the way out of the chaos. J Steroid Biochem Mol Biol 2017; 173: 117–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

