Evaluating Ga-68 Peptide Conjugates for Targeting VPAC Receptors: Stability and Pharmacokinetics
- PMID: 29802552
- PMCID: PMC6487500
- DOI: 10.1007/s11307-018-1207-x
Evaluating Ga-68 Peptide Conjugates for Targeting VPAC Receptors: Stability and Pharmacokinetics
Abstract
Purpose: In recent years, considerable progress has been made in the use of gallium-68 labeled receptor-specific peptides for imaging oncologic diseases. The objective was to examine the stability and pharmacokinetics of [68Ga]NODAGA and DOTA-peptide conjugate targeting VPAC [combined for vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP)] receptors on tumor cells.
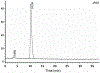
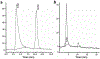
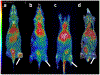
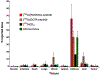
Procedures: A VPAC receptor-specific peptide was chosen as a model peptide and conjugated to NODAGA and DOTA via solid-phase synthesis. The conjugates were characterized by HPLC and MALDI-TOF. Following Ga-68 chelation, the radiochemical purity of Ga-68 labeled peptide conjugate was determined by radio-HPLC. The stability was tested against transmetallation using 100 nM Fe3+/Zn2+/Ca2+ ionic solution and against transchelation using 200 μM DTPA solution. The ex vivo and in vivo stability of the Ga-68 labeled peptide conjugate was tested in mouse plasma and urine. Receptor specificity was determined ex vivo by cell binding assays using human breast cancer BT474 cells. Positron emission tomography (PET)/X-ray computed tomography (CT) imaging, tissue distribution, and blocking studies were performed in mice bearing BT474 xenografts.
Results: The chemical and radiochemical purity was greater than 95 % and both conjugates were stable against transchelation and transmetallation. Ex vivo stability at 60 min showed that the NODAGA-peptide-bound Ga-68 reduced to 42.1 ± 3.7 % (in plasma) and 37.4 ± 2.9 % (in urine), whereas the DOTA-peptide-bound Ga-68 was reduced to 1.2 ± 0.3 % (in plasma) and 4.2 ± 0.4 % (in urine) at 60 min. Similarly, the in vivo stability for [68Ga]NODAGA-peptide was decreased to 2.1 ± 0.2 % (in plasma) and 2.2 ± 0.4 % (in urine). For [68Ga]DOTA-peptide, it was decreased to 1.4 ± 0.3 % (in plasma) and 1.2 ± 0.4 % (in urine) at 60 min. The specific BT474 cell binding was 53.9 ± 0.8 % for [68Ga]NODAGA-peptide, 25.8 ± 1.4 % for [68Ga]-DOTA-peptide, and 18.8 ± 2.5 % for [68Ga]GaCl3 at 60 min. Inveon microPET/CT imaging at 1 h post-injection showed significantly (p < 0.05) higher tumor to muscle (T/M) ratio for [68Ga]NODAGA-peptide (3.4 ± 0.3) as compared to [68Ga]DOTA-peptide (1.8 ± 0.6). For [68Ga]GaCl3 and blocked mice, their ratios were 1.5 ± 0.6 and 1.5 ± 0.3 respectively. The tissue distributions data were similar to the PET imaging data.
Conclusion: NODAGA is superior to DOTA in terms of radiolabeling kinetics. The method of radiolabeling was reproducible and yielded higher specific activity. Although both agents have relatively low in vivo stability, PET/CT imaging studies delineated BC tumors with [68Ga]NODAGA-peptide, but not with [68Ga]DOTA-peptide.
Keywords: Chelating agents; Gallium-68; Molecular imaging; Radiochemistry; Tumor imaging.
Conflict of interest statement
Conflict of Interest
Mathew L. Thakur is consultant to NuView and Zevacor, Inc. No other potential conflicts of interest relevant to this article are reported.
Figures


Similar articles
-
68Ga-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers.Nucl Med Biol. 2012 May;39(4):560-9. doi: 10.1016/j.nucmedbio.2011.10.011. Epub 2011 Dec 14. Nucl Med Biol. 2012. PMID: 22172391
-
Evaluation of a PACAP Peptide Analogue Labeled with (68)Ga Using Two Different Chelating Agents.Cancer Biother Radiopharm. 2016 Feb;31(1):29-36. doi: 10.1089/cbr.2015.1947. Cancer Biother Radiopharm. 2016. PMID: 26844850 Free PMC article.
-
(64)Cu- and (68)Ga-Based PET Imaging of Folate Receptor-Positive Tumors: Development and Evaluation of an Albumin-Binding NODAGA-Folate.Mol Pharm. 2016 Jun 6;13(6):1979-87. doi: 10.1021/acs.molpharmaceut.6b00143. Epub 2016 May 4. Mol Pharm. 2016. PMID: 27145400
-
68Ga-Labeled β-aminoalanine, γ-aminohomoalanine, and ε-aminolysine conjugates of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid.2011 Aug 29 [updated 2011 Oct 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Aug 29 [updated 2011 Oct 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22013604 Free Books & Documents. Review.
-
68Ga-1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid-1,2-diaminoethane-γ-5,8-dideazfolic acid (P3238).2012 Jun 14 [updated 2012 Sep 20]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Jun 14 [updated 2012 Sep 20]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23016165 Free Books & Documents. Review.
Cited by
-
Peptide-based positron emission tomography probes: current strategies for synthesis and radiolabelling.RSC Med Chem. 2023 Jan 6;14(4):592-623. doi: 10.1039/d2md00397j. eCollection 2023 Apr 26. RSC Med Chem. 2023. PMID: 37122545 Free PMC article. Review.
-
Demonstration of the Early Cardiac Bioavailability of a Non-Specific Cell-Targeted Peptide Using Radionuclide-Based Imaging In Vivo.Pharmaceuticals (Basel). 2023 May 31;16(6):824. doi: 10.3390/ph16060824. Pharmaceuticals (Basel). 2023. PMID: 37375771 Free PMC article.
-
Development of Cobalt-Binding Peptide Chelate from Human Serum Albumin: Cobalt-Binding Properties and Stability.Int J Mol Sci. 2022 Jan 10;23(2):719. doi: 10.3390/ijms23020719. Int J Mol Sci. 2022. PMID: 35054904 Free PMC article.
-
Chelator-Free/Chelator-Mediated Radiolabeling of Colloidally Stabilized Iron Oxide Nanoparticles for Biomedical Imaging.Nanomaterials (Basel). 2021 Jun 25;11(7):1677. doi: 10.3390/nano11071677. Nanomaterials (Basel). 2021. PMID: 34202370 Free PMC article.
-
Preclinical Advances in Theranostics for the Different Molecular Subtypes of Breast Cancer.Front Pharmacol. 2021 Apr 27;12:627693. doi: 10.3389/fphar.2021.627693. eCollection 2021. Front Pharmacol. 2021. PMID: 33986665 Free PMC article. Review.
References
-
- Al-Nahhas A, Win Z, Szyszko T et al. (2007) Gallium-68 PET: a new frontier in receptor cancer imaging. Anticancer Res 27:4087–4094 - PubMed
-
- Ambrosini V, Campana D, Tomassetti P et al. (2011) PET/CT with [68Ga]gallium-DOTA-peptides in NET: an overview. Eur J Radiol 80:116 - PubMed
-
- Baum RP, Kulkarni HR, Carreras C (2012) Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med 42:190–207 - PubMed
-
- Weiner RE, Thakur ML (2005) Radiolabeled peptides in oncology: role in diagnosis and treatment. BioDrugs 19:145–163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

