Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound
- PMID: 29802017
- PMCID: PMC6127501
- DOI: 10.1016/j.ymthe.2018.04.021
Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound
Abstract
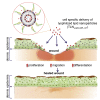
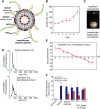
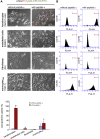
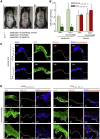
Lyophilized keratinocyte-targeted nanocarriers (TLNκ) loaded with locked nucleic acid (LNA) modified anti-miR were developed for topical application to full thickness burn injury. TLNκ were designed to selectively deliver LNA-anti-miR-107 to keratinocytes using the peptide sequence ASKAIQVFLLAG. TLNκ employed DOTAP/DODAP combination pH-responsive lipid components to improve endosomal escape. To minimize interference of clearance by non-targeted cells, especially immune cells in the acute wound microenvironment, surface charge was neutralized. Lyophilization was performed to extend the shelf life of the lipid nanoparticles (LNPs). Encapsulation efficiency of anti-miR in lyophilized TLNκ was estimated to be 96.54%. Cargo stability of lyophilized TLNκ was tested. After 9 days of loading with anti-miR-210, TLNκ was effective in lowering abundance of the hypoxamiR miR-210 in keratinocytes challenged with hypoxia. Keratinocyte uptake of DiD-labeled TLNκ was selective and exceeded 90% within 4 hr. Topical application of hydrogel-dispersed lyophilized TLNκ encapsulating LNA anti-miR-107 twice a week significantly accelerated wound closure and restoration of skin barrier function. TLNκ/anti-miR-107 application depleted miR-107 and upregulated dicer expression, which accelerated differentiation of keratinocytes. Expression of junctional proteins such as claudin-1, loricrin, filaggrin, ZO-1, and ZO-2 were significantly upregulated following TLNκ/anti-miR-107 treatment. These LNPs are promising as topical therapeutic agents in the management of burn injury.
Keywords: burn wound; drug targeting; microRNAs; nanoparticles; wound healing.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
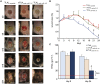
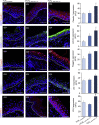
Comment in
-
Nanotechnology in Wound Care: One Step Closer to the Clinic.Mol Ther. 2018 Sep 5;26(9):2085-2086. doi: 10.1016/j.ymthe.2018.08.008. Epub 2018 Aug 16. Mol Ther. 2018. PMID: 30121229 Free PMC article. No abstract available.
Similar articles
-
Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration.Theranostics. 2020 Aug 8;10(22):9970-9983. doi: 10.7150/thno.46639. eCollection 2020. Theranostics. 2020. PMID: 32929328 Free PMC article.
-
MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression.Diabetes. 2018 Aug;67(8):1627-1638. doi: 10.2337/db17-1238. Epub 2018 May 10. Diabetes. 2018. PMID: 29748291
-
AntihypoxamiR functionalized gramicidin lipid nanoparticles rescue against ischemic memory improving cutaneous wound healing.Nanomedicine. 2016 Oct;12(7):1827-1831. doi: 10.1016/j.nano.2016.03.004. Epub 2016 Mar 29. Nanomedicine. 2016. PMID: 27033464 Free PMC article.
-
MicroRNAs involved in human skin burns, wound healing and scarring.Wound Repair Regen. 2023 Jul-Aug;31(4):439-453. doi: 10.1111/wrr.13100. Epub 2023 Jun 2. Wound Repair Regen. 2023. PMID: 37268303 Review.
-
Lipid Engineered Nanoparticle Therapy for Burn Wound Treatment.Curr Pharm Biotechnol. 2022;23(12):1449-1459. doi: 10.2174/1389201022666210823110532. Curr Pharm Biotechnol. 2022. PMID: 34425743 Review.
Cited by
-
A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages.Sci Rep. 2019 Oct 4;9(1):14293. doi: 10.1038/s41598-019-49435-z. Sci Rep. 2019. PMID: 31586077 Free PMC article.
-
Skin Transcriptome of Middle-Aged Women Supplemented With Natural Herbo-mineral Shilajit Shows Induction of Microvascular and Extracellular Matrix Mechanisms.J Am Coll Nutr. 2019 Aug;38(6):526-536. doi: 10.1080/07315724.2018.1564088. Epub 2019 Jun 4. J Am Coll Nutr. 2019. PMID: 31161927 Free PMC article. Clinical Trial.
-
Analysis of Keratinocytic Exosomes from Diabetic and Nondiabetic Mice by Charge Detection Mass Spectrometry.Anal Chem. 2022 Jun 28;94(25):8909-8918. doi: 10.1021/acs.analchem.2c00453. Epub 2022 Jun 14. Anal Chem. 2022. PMID: 35699514 Free PMC article.
-
Smart and versatile biomaterials for cutaneous wound healing.Biomater Res. 2023 Sep 16;27(1):87. doi: 10.1186/s40824-023-00426-2. Biomater Res. 2023. PMID: 37717028 Free PMC article. Review.
-
Ketoconazole resistant Candida albicans is sensitive to a wireless electroceutical wound care dressing.Bioelectrochemistry. 2021 Dec;142:107921. doi: 10.1016/j.bioelechem.2021.107921. Epub 2021 Aug 4. Bioelectrochemistry. 2021. PMID: 34419917 Free PMC article.
References
-
- Ashton S., Song Y.H., Nolan J., Cadogan E., Murray J., Odedra R., Foster J., Hall P.A., Low S., Taylor P. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci. Transl. Med. 2016;8:325ra17. - PubMed
-
- Dritschilo A., Huang C.H., Rudin C.M., Marshall J., Collins B., Dul J.L., Zhang C., Kumar D., Gokhale P.C., Ahmad A. Phase I study of liposome-encapsulated c-raf antisense oligodeoxyribonucleotide infusion in combination with radiation therapy in patients with advanced malignancies. Clin. Cancer Res. 2006;12:1251–1259. - PubMed
-
- Elazar V., Adwan H., Bäuerle T., Rohekar K., Golomb G., Berger M.R. Sustained delivery and efficacy of polymeric nanoparticles containing osteopontin and bone sialoprotein antisenses in rats with breast cancer bone metastasis. Int. J. Cancer. 2010;126:1749–1760. - PubMed
-
- Tabernero J., Shapiro G.I., LoRusso P.M., Cervantes A., Schwartz G.K., Weiss G.J., Paz-Ares L., Cho D.C., Infante J.R., Alsina M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

