A-Kinase-Anchoring Protein (AKAP150) is expressed in Astrocytes and Upregulated in Response to Ischemia
- PMID: 29800717
- PMCID: PMC6238626
- DOI: 10.1016/j.neuroscience.2018.05.019
A-Kinase-Anchoring Protein (AKAP150) is expressed in Astrocytes and Upregulated in Response to Ischemia
Abstract
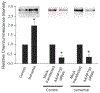
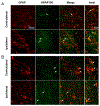
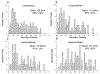
A-kinase-anchoring proteins, AKAPs, are scaffolding proteins that associate with kinases and phosphatases, and direct them to a specific submembrane site to coordinate signaling events. AKAP150, a rodent ortholog of human AKAP79, has been extensively studied in neurons, but very little is known about the localization and function of AKAP150 in astrocytes, the major cell type in brain. Thus, in this study, we assessed the localization of AKAP150 in astrocytes and elucidated its role during physiological and ischemic conditions. Herein, we demonstrate that AKAP150 is localized in astrocytes and is up-regulated during ischemia both in vitro and in vivo. Knock-down of AKAP150 by RNAi depolarizes the astrocytic membrane potential and substantially reduces by 80% the ability of astrocytes to take up extracellular potassium during ischemic conditions. Therefore, upregulation of AKAP150 during ischemia preserves potassium conductance and the associated hyperpolarized membrane potential of astrocytes; properties of astrocytes needed to maintain extracellular brain homeostasis. Taken together, these data suggest that AKAP150 may play a pivotal role in the neuroprotective mechanism of astrocytes during pathological conditions.
Keywords: cortical astrocytes; membrane potential; middle cerebral artery occlusion; potassium uptake; siRNA.
Copyright © 2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
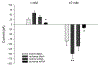
Similar articles
-
Decreased expression of A-kinase anchoring protein 150 in GT1 neurons decreases neuron excitability and frequency of intrinsic gonadotropin-releasing hormone pulses.Endocrinology. 2010 Jan;151(1):281-90. doi: 10.1210/en.2009-0894. Epub 2009 Nov 3. Endocrinology. 2010. PMID: 19887564 Free PMC article.
-
Up-regulation of TREK-2 potassium channels in cultured astrocytes requires de novo protein synthesis: relevance to localization of TREK-2 channels in astrocytes after transient cerebral ischemia.PLoS One. 2015 Apr 17;10(4):e0125195. doi: 10.1371/journal.pone.0125195. eCollection 2015. PLoS One. 2015. PMID: 25886567 Free PMC article.
-
AKAP150 involved in paclitaxel-induced neuropathic pain via inhibiting CN/NFAT2 pathway and downregulating IL-4.Brain Behav Immun. 2018 Feb;68:158-168. doi: 10.1016/j.bbi.2017.10.015. Epub 2017 Oct 19. Brain Behav Immun. 2018. PMID: 29056557
-
Astrocytes and ischemic tolerance.Neurosci Res. 2018 Jan;126:53-59. doi: 10.1016/j.neures.2017.11.013. Epub 2017 Dec 7. Neurosci Res. 2018. PMID: 29225139 Review.
-
Altered Homeostatic Functions in Reactive Astrocytes and Their Potential as a Therapeutic Target After Brain Ischemic Injury.Curr Pharm Des. 2017;23(33):5056-5074. doi: 10.2174/1381612823666170710161858. Curr Pharm Des. 2017. PMID: 28699523 Review.
Cited by
-
β-COP Regulates TWIK1/TREK1 Heterodimeric Channel-Mediated Passive Conductance in Astrocytes.Cells. 2022 Oct 21;11(20):3322. doi: 10.3390/cells11203322. Cells. 2022. PMID: 36291187 Free PMC article.
-
Role of TRP ion channels in cerebral circulation and neurovascular communication.Neurosci Lett. 2021 Nov 20;765:136258. doi: 10.1016/j.neulet.2021.136258. Epub 2021 Sep 22. Neurosci Lett. 2021. PMID: 34560190 Free PMC article. Review.
References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999), Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215. - PubMed
-
- Bolton S, Greenwood K, Hamilton N, Butt AM (2006), Regulation of the astrocyte resting membrane potential by cyclic AMP and protein kinase A. Glia 54:316–328. - PubMed
-
- Carnegie GK, Scott JD (2003), A-kinase anchoring proteins and neuronal signaling mechanisms. Genes Dev 17:1557–1568. - PubMed
-
- Carr DW, Stofko-Han RE, Fraser IDC, Cone RD, Scott JD (1992), Localization of the cAMP-dependent protein densities by A-kinase anchoring proteins. J Biol Chem 267:16816–1623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

