Regulator of calcineurin 1 differentially regulates TLR-dependent MyD88 and TRIF signaling pathways
- PMID: 29799862
- PMCID: PMC5969770
- DOI: 10.1371/journal.pone.0197491
Regulator of calcineurin 1 differentially regulates TLR-dependent MyD88 and TRIF signaling pathways
Abstract
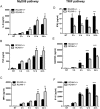
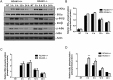
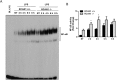
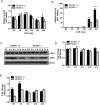
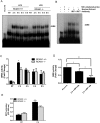
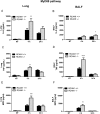
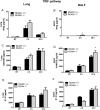
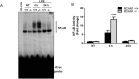
Toll-like receptors (TLRs) recognize the conserved molecular patterns in microorganisms and trigger myeloid differentiation primary response 88 (MyD88) and/or TIR-domain-containing adapter-inducing interferon-β (TRIF) pathways that are critical for host defense against microbial infection. However, the molecular mechanisms that govern TLR signaling remain incompletely understood. Regulator of calcineurin-1 (RCAN1), a small evolutionarily conserved protein that inhibits calcineurin phosphatase activity, suppresses inflammation during Pseudomonas aeruginosa infection. Here, we define the roles for RCAN1 in P. aeruginosa lipopolysaccharide (LPS)-activated TLR4 signaling. We compared the effects of P. aeruginosa LPS challenge on bone marrow-derived macrophages from both wild-type and RCAN1-deficient mice and found that RCAN1 deficiency increased the MyD88-NF-κB-mediated cytokine production (IL-6, TNF and MIP-2), whereas TRIF-interferon-stimulated response elements (ISRE)-mediated cytokine production (IFNβ, RANTES and IP-10) was suppressed. RCAN1 deficiency caused increased IκBα phosphorylation and NF-κB activity in the MyD88-dependent pathway, but impaired ISRE activation and reduced IRF7 expression in the TRIF-dependent pathway. Complementary studies of a mouse model of P. aeruginosa LPS-induced acute pneumonia confirmed that RCAN1-deficient mice displayed greatly enhanced NF-κB activity and MyD88-NF-κB-mediated cytokine production, which correlated with enhanced pulmonary infiltration of neutrophils. By contrast, RCAN1 deficiency had little effect on the TRIF pathway in vivo. These findings demonstrate a novel regulatory role of RCAN1 in TLR signaling, which differentially regulates MyD88 and TRIF pathways.
Conflict of interest statement
The authors have no competing interests related to this manuscript.
Figures
Similar articles
-
Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells.J Pineal Res. 2012 Nov;53(4):325-34. doi: 10.1111/j.1600-079X.2012.01002.x. Epub 2012 Apr 27. J Pineal Res. 2012. PMID: 22537289
-
Calcineurin negatively regulates TLR-mediated activation pathways.J Immunol. 2007 Oct 1;179(7):4598-607. doi: 10.4049/jimmunol.179.7.4598. J Immunol. 2007. PMID: 17878357
-
AKT1 distinctively suppresses MyD88-depenedent and TRIF-dependent Toll-like receptor signaling in a kinase activity-independent manner.Cell Signal. 2018 Mar;43:32-39. doi: 10.1016/j.cellsig.2017.12.002. Epub 2017 Dec 11. Cell Signal. 2018. PMID: 29242168
-
TIR domain-containing adaptors define the specificity of TLR signaling.Mol Immunol. 2004 Feb;40(12):861-8. doi: 10.1016/j.molimm.2003.10.006. Mol Immunol. 2004. PMID: 14698224 Review.
-
Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury.Int J Exp Pathol. 2007 Dec;88(6):387-91. doi: 10.1111/j.1365-2613.2007.00566.x. Int J Exp Pathol. 2007. PMID: 18039275 Free PMC article. Review.
Cited by
-
Early Growth Response 1 Deficiency Protects the Host against Pseudomonas aeruginosa Lung Infection.Infect Immun. 2019 Dec 17;88(1):e00678-19. doi: 10.1128/IAI.00678-19. Print 2019 Dec 17. Infect Immun. 2019. PMID: 31611276 Free PMC article.
-
Long noncoding RNA MyD88 functions as a promising diagnostic biomarker in hepatocellular carcinoma.Front Endocrinol (Lausanne). 2023 Jan 30;14:938102. doi: 10.3389/fendo.2023.938102. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36793272 Free PMC article.
-
Eicosapentaenoic acid loaded silica nanoemulsion attenuates hepatic inflammation through the enhancement of cell membrane components.Biol Proced Online. 2022 Sep 7;24(1):11. doi: 10.1186/s12575-022-00173-z. Biol Proced Online. 2022. PMID: 36071378 Free PMC article.
-
Baishouwu Extract Suppresses the Development of Hepatocellular Carcinoma via TLR4/MyD88/NF-κB Pathway.Front Pharmacol. 2019 Apr 24;10:389. doi: 10.3389/fphar.2019.00389. eCollection 2019. Front Pharmacol. 2019. PMID: 31068809 Free PMC article.
-
NF-κB signaling pathway inhibition suppresses hippocampal neuronal apoptosis and cognitive impairment via RCAN1 in neonatal rats with hypoxic-ischemic brain damage.Cell Cycle. 2019 May;18(9):1001-1018. doi: 10.1080/15384101.2019.1608128. Epub 2019 May 3. Cell Cycle. 2019. Retraction in: Cell Cycle. 2022 Apr;21(7):764. doi: 10.1080/15384101.2022.2046806 PMID: 30990350 Free PMC article. Retracted.
References
-
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. . - PubMed
-
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461 doi: 10.3389/fimmu.2014.00461 . - DOI - PMC - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391 . - DOI - PubMed
-
- Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17(1):3–9. doi: 10.1165/ajrcmb.17.1.f132 . - DOI - PubMed
-
- Solis M, Romieu-Mourez R, Goubau D, Grandvaux N, Mesplede T, Julkunen I, et al. Involvement of TBK1 and IKKepsilon in lipopolysaccharide-induced activation of the interferon response in primary human macrophages. Eur J Immunol. 2007;37(2):528–39. doi: 10.1002/eji.200636090 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

