Efficacy of Vaginally Administered Gel Containing Emtricitabine and Tenofovir Against Repeated Rectal Simian Human Immunodeficiency Virus Exposures in Macaques
- PMID: 29788316
- PMCID: PMC6129108
- DOI: 10.1093/infdis/jiy301
Efficacy of Vaginally Administered Gel Containing Emtricitabine and Tenofovir Against Repeated Rectal Simian Human Immunodeficiency Virus Exposures in Macaques
Abstract
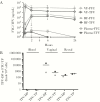
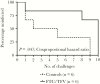
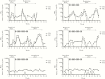
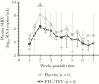
Vaginal microbicides containing antiretrovirals (ARVs) have shown to prevent vaginally acquired human immunodeficiency virus (HIV), but these products may not protect women who engage in anal sex. Intravaginal dosing with ARVs has shown to result in drug exposures in rectal tissues, thus raising the possibility of dual compartment protection. To test this concept, we investigated whether intravaginal dosing with emtricitabine (FTC)/tenofovir (TFV) gel, which fully protected macaques against repeated vaginal exposures to simian human immunodeficiency virus (SHIV), protects against rectal SHIV exposures. Pharmacokinetic studies revealed rapid distribution of FTC and TFV to rectal tissues and luminal fluids, albeit at concentrations 1-2 log10 lower than those in the vaginal compartment. Efficacy measurements against repeated rectal SHIV challenges demonstrated a 4.5-fold reduction in risk of infection in macaques that received intravaginal FTC/TFV compared to placebo gel (P = .047; log-rank test). These data support the concept of dual compartment protection by vaginal dosing and warrants developing ARV-based vaginal products with improved bidirectional dosing.
Figures
Similar articles
-
Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine.J Virol. 2009 Oct;83(20):10358-65. doi: 10.1128/JVI.01073-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656878 Free PMC article.
-
Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue.J Virol. 2012 Jan;86(2):718-25. doi: 10.1128/JVI.05842-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072766 Free PMC article.
-
Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques.Antimicrob Agents Chemother. 2012 Jan;56(1):103-9. doi: 10.1128/AAC.00597-11. Epub 2011 Oct 10. Antimicrob Agents Chemother. 2012. PMID: 21986823 Free PMC article.
-
Animal models of antiretroviral prophylaxis for HIV prevention.Curr Opin HIV AIDS. 2012 Nov;7(6):505-13. doi: 10.1097/COH.0b013e328358e484. Curr Opin HIV AIDS. 2012. PMID: 22964889 Review.
-
Rectal microbicide development.Curr Top Microbiol Immunol. 2014;383:117-36. doi: 10.1007/82_2013_325. Curr Top Microbiol Immunol. 2014. PMID: 23612991 Free PMC article. Review.
Cited by
-
Translational Models to Predict Target Concentrations for Pre-Exposure Prophylaxis in Women.AIDS Res Hum Retroviruses. 2022 Dec;38(12):909-923. doi: 10.1089/AID.2022.0057. Epub 2022 Oct 25. AIDS Res Hum Retroviruses. 2022. PMID: 36097755 Free PMC article. Review.
-
Combination Antiretroviral Therapy and Immunophenotype of Feline Immunodeficiency Virus.Viruses. 2023 Mar 24;15(4):822. doi: 10.3390/v15040822. Viruses. 2023. PMID: 37112803 Free PMC article.
-
Single dose topical inserts containing tenofovir alafenamide fumarate and elvitegravir provide pre- and post-exposure protection against vaginal SHIV infection in macaques.EBioMedicine. 2022 Dec;86:104361. doi: 10.1016/j.ebiom.2022.104361. Epub 2022 Nov 21. EBioMedicine. 2022. PMID: 36423375 Free PMC article.
-
Increases in HIV Incidence Following Receptive Anal Intercourse Among Women: A Systematic Review and Meta-analysis.AIDS Behav. 2020 Mar;24(3):667-681. doi: 10.1007/s10461-019-02651-0. AIDS Behav. 2020. PMID: 31486008 Free PMC article.
-
Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection.Pharmaceutics. 2024 Mar 11;16(3):384. doi: 10.3390/pharmaceutics16030384. Pharmaceutics. 2024. PMID: 38543278 Free PMC article.
References
-
- Coutinho C, Sarmento B, das Neves J. Targeted microbicides for preventing sexual HIV transmission. J Control Release 2017; 266:119–28. - PubMed
-
- Dobard C, Sharma S, Parikh UM et al. . Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med 2014; 6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

