HIV vaccine candidate activation of hypoxia and the inflammasome in CD14+ monocytes is associated with a decreased risk of SIVmac251 acquisition
- PMID: 29785023
- PMCID: PMC5992093
- DOI: 10.1038/s41591-018-0025-7
HIV vaccine candidate activation of hypoxia and the inflammasome in CD14+ monocytes is associated with a decreased risk of SIVmac251 acquisition
Abstract
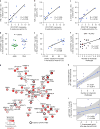
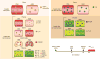
Qualitative differences in the innate and adaptive responses elicited by different HIV vaccine candidates have not been thoroughly investigated. We tested the ability of the Aventis Pasteur live recombinant canarypox vector (ALVAC)-SIV, DNA-SIV and Ad26-SIV vaccine prime modalities together with two ALVAC-SIV + gp120 protein boosts to reduce the risk of SIVmac251 acquisition in rhesus macaques. We found that the DNA and ALVAC prime regimens were effective, but the Ad26 prime was not. The activation of hypoxia and the inflammasome in CD14+CD16- monocytes, gut-homing CCR5-negative CD4+ T helper 2 (TH2) cells and antibodies to variable region 2 correlated with a decreased risk of SIVmac251 acquisition. By contrast, signal transducer and activator of transcription 3 activation in CD16+ monocytes was associated with an increased risk of virus acquisition. The Ad26 prime regimen induced the accumulation of CX3CR1+CD163+ macrophages in lymph nodes and of long-lasting CD4+ TH17 cells in the gut and lungs. Our data indicate that the selective engagement of monocyte subsets following a vaccine prime influences long-term immunity, uncovering an unexpected association of CD14+ innate monocytes with a reduced risk of SIVmac251 acquisition.
Conflict of interest statement
The US Government in conjunction with Sanofi Pasteur holds Patent 5766598: A Recombinant Attenuated ALVAC Canarypox virus Expression Vectors Containing Heterologous DNA Segments Encoding Lentiviral Gene, inventors E. Paoletti, J. Tartaglia and W. I. Cox, issued 16 June 1998, for the ALVAC vaccine. The US Government also holds Patent 7094408: Improved Immunogenicity Using a Combination of DNA and Vaccinia Virus Vector Vaccines, inventors G. Franchini, Z. Hel and G. Pavlakis, issued 22 August 2006. This patent is for the combination DNA and ALVAC poxvirus vaccines.
Figures




Similar articles
-
Expression of CD40L by the ALVAC-Simian Immunodeficiency Virus Vector Abrogates T Cell Responses in Macaques.J Virol. 2020 Feb 28;94(6):e01933-19. doi: 10.1128/JVI.01933-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31896599 Free PMC article.
-
Engagement of monocytes, NK cells, and CD4+ Th1 cells by ALVAC-SIV vaccination results in a decreased risk of SIVmac251 vaginal acquisition.PLoS Pathog. 2020 Mar 12;16(3):e1008377. doi: 10.1371/journal.ppat.1008377. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32163525 Free PMC article.
-
Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques.J Virol. 2019 Feb 5;93(4):e01687-18. doi: 10.1128/JVI.01687-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463978 Free PMC article.
-
A Mucosal Adenovirus Prime/Systemic Envelope Boost Vaccine Regimen Elicits Responses in Cervicovaginal and Alveolar Macrophages of Rhesus Macaques Associated With Delayed SIV Acquisition and B Cell Help.Front Immunol. 2020 Sep 30;11:571804. doi: 10.3389/fimmu.2020.571804. eCollection 2020. Front Immunol. 2020. PMID: 33117363 Free PMC article.
-
Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies.J Neurovirol. 2008 Aug;14(4):318-26. doi: 10.1080/13550280802132857. J Neurovirol. 2008. PMID: 18780233 Free PMC article. Review.
Cited by
-
Loss of HIV candidate vaccine efficacy in male macaques by mucosal nanoparticle immunization rescued by V2-specific response.Nat Commun. 2024 Oct 22;15(1):9102. doi: 10.1038/s41467-024-53359-2. Nat Commun. 2024. PMID: 39438480 Free PMC article.
-
Expression of CD40L by the ALVAC-Simian Immunodeficiency Virus Vector Abrogates T Cell Responses in Macaques.J Virol. 2020 Feb 28;94(6):e01933-19. doi: 10.1128/JVI.01933-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31896599 Free PMC article.
-
Myeloid Cell-Mediated Trained Innate Immunity in Mucosal AIDS Vaccine Development.Front Immunol. 2020 Feb 28;11:315. doi: 10.3389/fimmu.2020.00315. eCollection 2020. Front Immunol. 2020. PMID: 32184782 Free PMC article. Review.
-
Engineering Biomaterials to Direct Innate Immunity.Adv Ther (Weinh). 2019 Jun;2(6):1800157. doi: 10.1002/adtp.201800157. Epub 2019 Feb 27. Adv Ther (Weinh). 2019. PMID: 31236439 Free PMC article.
-
Monocyte-derived transcriptome signature indicates antibody-dependent cellular phagocytosis as a potential mechanism of vaccine-induced protection against HIV-1.Elife. 2021 Sep 17;10:e69577. doi: 10.7554/eLife.69577. Elife. 2021. PMID: 34533134 Free PMC article.
References
-
- Pitisuttithum P, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006;194:1661–1671. - PubMed
-
- Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

