A guide to using functional magnetic resonance imaging to study Alzheimer's disease in animal models
- PMID: 29784664
- PMCID: PMC5992611
- DOI: 10.1242/dmm.031724
A guide to using functional magnetic resonance imaging to study Alzheimer's disease in animal models
Abstract
Alzheimer's disease is a leading healthcare challenge facing our society today. Functional magnetic resonance imaging (fMRI) of the brain has played an important role in our efforts to understand how Alzheimer's disease alters brain function. Using fMRI in animal models of Alzheimer's disease has the potential to provide us with a more comprehensive understanding of the observations made in human clinical fMRI studies. However, using fMRI in animal models of Alzheimer's disease presents some unique challenges. Here, we highlight some of these challenges and discuss potential solutions for researchers interested in performing fMRI in animal models. First, we briefly summarize our current understanding of Alzheimer's disease from a mechanistic standpoint. We then overview the wide array of animal models available for studying this disease and how to choose the most appropriate model to study, depending on which aspects of the condition researchers seek to investigate. Finally, we discuss the contributions of fMRI to our understanding of Alzheimer's disease and the issues to consider when designing fMRI studies for animal models, such as differences in brain activity based on anesthetic choice and ways to interrogate more specific questions in rodents beyond those that can be addressed in humans. The goal of this article is to provide information on the utility of fMRI, and approaches to consider when using fMRI, for studies of Alzheimer's disease in animal models.
Keywords: Alzheimer's disease; Anesthesia; Mouse models; Resting state; fMRI.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
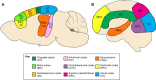
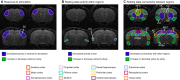

Similar articles
-
Identifying patients with Alzheimer's disease using resting-state fMRI and graph theory.Clin Neurophysiol. 2015 Nov;126(11):2132-41. doi: 10.1016/j.clinph.2015.02.060. Epub 2015 Apr 1. Clin Neurophysiol. 2015. PMID: 25907414
-
A survey on applications and analysis methods of functional magnetic resonance imaging for Alzheimer's disease.J Neurosci Methods. 2019 Apr 1;317:121-140. doi: 10.1016/j.jneumeth.2018.12.012. Epub 2018 Dec 26. J Neurosci Methods. 2019. PMID: 30593787 Review.
-
Multi-label classification of Alzheimer's disease stages from resting-state fMRI-based correlation connectivity data and deep learning.Comput Biol Med. 2022 Dec;151(Pt A):106240. doi: 10.1016/j.compbiomed.2022.106240. Epub 2022 Nov 9. Comput Biol Med. 2022. PMID: 36423532
-
Differences between Alzheimer's disease and dementia with Lewy bodies: an fMRI study of task-related brain activity.Brain. 2006 Jul;129(Pt 7):1780-8. doi: 10.1093/brain/awl102. Epub 2006 May 2. Brain. 2006. PMID: 16670180
-
Regional homogeneity, functional connectivity and imaging markers of Alzheimer's disease: a review of resting-state fMRI studies.Neuropsychologia. 2008;46(6):1648-56. doi: 10.1016/j.neuropsychologia.2008.01.027. Epub 2008 Feb 14. Neuropsychologia. 2008. PMID: 18346763 Review.
Cited by
-
An open database of resting-state fMRI in awake rats.Neuroimage. 2020 Oct 15;220:117094. doi: 10.1016/j.neuroimage.2020.117094. Epub 2020 Jun 28. Neuroimage. 2020. PMID: 32610063 Free PMC article.
-
Application of Optogenetics in Neurodegenerative Diseases.Cell Mol Neurobiol. 2024 Jul 26;44(1):57. doi: 10.1007/s10571-024-01486-1. Cell Mol Neurobiol. 2024. PMID: 39060759 Free PMC article. Review.
-
A network pharmacology approach to uncover the key ingredients in Ginkgo Folium and their anti-Alzheimer's disease mechanisms.Aging (Albany NY). 2021 Jul 27;13(14):18993-19012. doi: 10.18632/aging.203348. Epub 2021 Jul 27. Aging (Albany NY). 2021. PMID: 34315132 Free PMC article.
-
Disease modelling in human organoids.Dis Model Mech. 2019 Jul 29;12(7):dmm039347. doi: 10.1242/dmm.039347. Dis Model Mech. 2019. PMID: 31383635 Free PMC article. Review.
-
Natural Corynanthe-Type Cholinesterase Inhibitors from Malaysian Uncaria attenuata Korth.: Isolation, Characterization, In Vitro and In Silico Studies.Metabolites. 2023 Mar 7;13(3):390. doi: 10.3390/metabo13030390. Metabolites. 2023. PMID: 36984830 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

