Impact of current and scaled-up levels of hepatitis C prevention and treatment interventions for people who inject drugs in three UK settings-what is required to achieve the WHO's HCV elimination targets?
- PMID: 29774607
- PMCID: PMC6175066
- DOI: 10.1111/add.14217
Impact of current and scaled-up levels of hepatitis C prevention and treatment interventions for people who inject drugs in three UK settings-what is required to achieve the WHO's HCV elimination targets?
Abstract
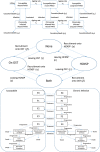
Aims: To estimate the impact of existing high-coverage needle and syringe provision (HCNSP, defined as obtaining more than one sterile needle and syringe per injection reported) and opioid substitution therapy (OST) on hepatitis C virus (HCV) transmission among people who inject drugs (PWID) in three UK settings and to determine required scale-up of interventions, including HCV treatment, needed to reach the World Health Organization (WHO) target of reducing HCV incidence by 90% by 2030.
Design: HCV transmission modelling using UK empirical estimates for effect of OST and/or HCNSP on individual risk of HCV acquisition.
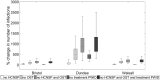
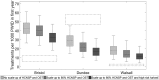
Setting and participants: Three UK cities with varying chronic HCV prevalence (Bristol 45%, Dundee 26%, Walsall 19%), OST (72-81%) and HCNSP coverage (28-56%).
Measurements: Relative change in new HCV infections throughout 2016-30 if current interventions were stopped. Scale-up of HCNSP, OST and HCV treatment required to achieve the WHO elimination target.
Findings: Removing HCNSP or OST would increase the number of new HCV infections throughout 2016 to 2030 by 23-64 and 92-483%, respectively. Conversely, scaling-up these interventions to 80% coverage could achieve a 29 or 49% reduction in Bristol and Walsall, respectively, whereas Dundee may achieve a 90% decrease in incidence with current levels of intervention because of existing high levels of HCV treatment (47-58 treatments per 1000 PWID). If OST and HCNSP are scaled-up, Walsall and Bristol can achieve the same impact by treating 14 or 40 per 1000 PWID annually, respectively (currently two and nine treatments per 1000 PWID), while 18 and 43 treatments per 1000 PWID would be required if OST and HCNSP are not scaled-up.
Conclusions: Current opioid substitution therapy and high-coverage needle and syringe provision coverage is averting substantial hepatitis C transmission in the United Kingdom. Maintaining this coverage while getting current drug injectors onto treatment can reduce incidence by 90% by 2030.
Keywords: HCV treatment scale-up; hepatitis C virus; mathematical model; needle and syringe provision; opioid substitution therapy; people who inject drugs.
© 2018 The Authors. Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures

Similar articles
-
Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy.Clin Infect Dis. 2013 Aug;57 Suppl 2(Suppl 2):S39-45. doi: 10.1093/cid/cit296. Clin Infect Dis. 2013. PMID: 23884064 Free PMC article.
-
Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe.J Hepatol. 2018 Mar;68(3):402-411. doi: 10.1016/j.jhep.2017.10.010. Epub 2018 Jan 8. J Hepatol. 2018. PMID: 29080808 Free PMC article.
-
Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012021. doi: 10.1002/14651858.CD012021.pub2. Cochrane Database Syst Rev. 2017. PMID: 28922449 Free PMC article. Review.
-
Modelling the impact of a national scale-up of interventions on hepatitis C virus transmission among people who inject drugs in Scotland.Addiction. 2018 Nov;113(11):2118-2131. doi: 10.1111/add.14267. Epub 2018 Jul 10. Addiction. 2018. PMID: 29781207 Free PMC article.
-
Assessing the impact and cost-effectiveness of needle and syringe provision and opioid substitution therapy on hepatitis C transmission among people who inject drugs in the UK: an analysis of pooled data sets and economic modelling.Southampton (UK): NIHR Journals Library; 2017 Sep. Southampton (UK): NIHR Journals Library; 2017 Sep. PMID: 28968044 Free Books & Documents. Review.
Cited by
-
Has the HCV cascade of care changed among people who inject drugs in England since the introduction of direct-acting antivirals?Int J Drug Policy. 2024 Jan 12:104324. doi: 10.1016/j.drugpo.2024.104324. Online ahead of print. Int J Drug Policy. 2024. PMID: 38218700 Free PMC article.
-
Methods and indicators to validate country reductions in incidence of hepatitis C virus infection to elimination levels set by WHO.Lancet Gastroenterol Hepatol. 2022 Apr;7(4):353-366. doi: 10.1016/S2468-1253(21)00311-3. Epub 2022 Feb 3. Lancet Gastroenterol Hepatol. 2022. PMID: 35122713 Free PMC article. Review.
-
'I just never wanted them to feel uncomfortable': Barriers to pharmacy-based identification and treatment of hepatitis C in Victoria, Canada.Can Liver J. 2024 May 8;7(2):257-272. doi: 10.3138/canlivj-2023-0016. eCollection 2024 May. Can Liver J. 2024. PMID: 38746863 Free PMC article.
-
Randomized feasibility trial of directly observed versus unobserved hepatitis C treatment with ledipasvir-sofosbuvir among people who inject drugs.PLoS One. 2019 Jun 3;14(6):e0217471. doi: 10.1371/journal.pone.0217471. eCollection 2019. PLoS One. 2019. PMID: 31158245 Free PMC article. Clinical Trial.
-
Uptake and predictors of direct-acting antiviral treatment for hepatitis C among people receiving opioid agonist therapy in Sweden and Norway: a drug utilization study from 2014 to 2017.Subst Abuse Treat Prev Policy. 2020 Jun 30;15(1):44. doi: 10.1186/s13011-020-00286-2. Subst Abuse Treat Prev Policy. 2020. PMID: 32605625 Free PMC article.
References
-
- World Health Organization (WHO) . Combating Hepatitis B and C to Reach Elimination by 2030. Geneva: WHO; 2016.
-
- Public Health England . Hepatitis C in the UK 2015 report. London, UK: Public Health England; 2015.
-
- Harris R. J., Ramsay M., Hope V. D., Brant L., Hickman M., Foster G. R. et al Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health 2012; 22: 187–192. - PubMed
-
- Palmateer N., Kimber J., Hickman M., Hutchinson S., Rhodes T., Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 2010; 105: 844–859. - PubMed
-
- Turner K. M., Hutchinson S., Vickerman P., Hope V., Craine N., Palmateer N. et al The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106: 1978–1988. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

