STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma
- PMID: 29773717
- PMCID: PMC6030433
- DOI: 10.1158/2159-8290.CD-18-0099
STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma
Abstract
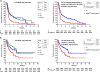
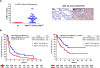
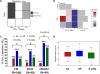
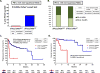
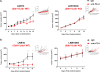
KRAS is the most common oncogenic driver in lung adenocarcinoma (LUAC). We previously reported that STK11/LKB1 (KL) or TP53 (KP) comutations define distinct subgroups of KRAS-mutant LUAC. Here, we examine the efficacy of PD-1 inhibitors in these subgroups. Objective response rates to PD-1 blockade differed significantly among KL (7.4%), KP (35.7%), and K-only (28.6%) subgroups (P < 0.001) in the Stand Up To Cancer (SU2C) cohort (174 patients) with KRAS-mutant LUAC and in patients treated with nivolumab in the CheckMate-057 phase III trial (0% vs. 57.1% vs. 18.2%; P = 0.047). In the SU2C cohort, KL LUAC exhibited shorter progression-free (P < 0.001) and overall (P = 0.0015) survival compared with KRASMUT;STK11/LKB1WT LUAC. Among 924 LUACs, STK11/LKB1 alterations were the only marker significantly associated with PD-L1 negativity in TMBIntermediate/High LUAC. The impact of STK11/LKB1 alterations on clinical outcomes with PD-1/PD-L1 inhibitors extended to PD-L1-positive non-small cell lung cancer. In Kras-mutant murine LUAC models, Stk11/Lkb1 loss promoted PD-1/PD-L1 inhibitor resistance, suggesting a causal role. Our results identify STK11/LKB1 alterations as a major driver of primary resistance to PD-1 blockade in KRAS-mutant LUAC.Significance: This work identifies STK11/LKB1 alterations as the most prevalent genomic driver of primary resistance to PD-1 axis inhibitors in KRAS-mutant lung adenocarcinoma. Genomic profiling may enhance the predictive utility of PD-L1 expression and tumor mutation burden and facilitate establishment of personalized combination immunotherapy approaches for genomically defined LUAC subsets. Cancer Discov; 8(7); 822-35. ©2018 AACR.See related commentary by Etxeberria et al., p. 794This article is highlighted in the In This Issue feature, p. 781.
©2018 American Association for Cancer Research.
Conflict of interest statement
Declaration of Interests
All other authors declare no competing financial interests.
Figures

Comment in
-
Epistatic Oncogenic Interactions Determine Cancer Susceptibility to Immunotherapy.Cancer Discov. 2018 Jul;8(7):794-796. doi: 10.1158/2159-8290.CD-18-0573. Cancer Discov. 2018. PMID: 29967073
Similar articles
-
STK11/LKB1 alterations worsen the poor prognosis of KRAS mutated early-stage non-squamous non-small cell lung carcinoma, results based on the phase 2 IFCT TASTE trial.Lung Cancer. 2024 Apr;190:107508. doi: 10.1016/j.lungcan.2024.107508. Epub 2024 Feb 19. Lung Cancer. 2024. PMID: 38428265 Clinical Trial.
-
The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity.Cancer Lett. 2020 Feb 1;470:95-105. doi: 10.1016/j.canlet.2019.10.027. Epub 2019 Oct 20. Cancer Lett. 2020. PMID: 31644929
-
Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer.Cancer. 2019 Apr 1;125(7):1038-1049. doi: 10.1002/cncr.31871. Epub 2018 Dec 11. Cancer. 2019. PMID: 30548240
-
Efficacy of immunotherapy in KRAS-mutant non-small-cell lung cancer with comutations.Immunotherapy. 2021 Aug;13(11):941-952. doi: 10.2217/imt-2021-0090. Epub 2021 Jun 11. Immunotherapy. 2021. PMID: 34114474 Review.
-
Modulating Tumor Microenvironment: A Review on STK11 Immune Properties and Predictive vs Prognostic Role for Non-small-cell Lung Cancer Immunotherapy.Curr Treat Options Oncol. 2021 Sep 15;22(11):96. doi: 10.1007/s11864-021-00891-8. Curr Treat Options Oncol. 2021. PMID: 34524570 Review.
Cited by
-
Association of MUC19 Mutation With Clinical Benefits of Anti-PD-1 Inhibitors in Non-small Cell Lung Cancer.Front Oncol. 2021 Mar 22;11:596542. doi: 10.3389/fonc.2021.596542. eCollection 2021. Front Oncol. 2021. PMID: 33828970 Free PMC article.
-
Primary Resistance to Immune Checkpoint Blockade in an STK11/TP53/KRAS-Mutant Lung Adenocarcinoma with High PD-L1 Expression.Onco Targets Ther. 2020 Sep 7;13:8901-8905. doi: 10.2147/OTT.S272013. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982282 Free PMC article.
-
Targeting KRAS Mutant Non-Small-Cell Lung Cancer: Past, Present and Future.Int J Mol Sci. 2020 Jun 17;21(12):4325. doi: 10.3390/ijms21124325. Int J Mol Sci. 2020. PMID: 32560574 Free PMC article. Review.
-
EZH2 Inhibition Promotes Tumor Immunogenicity in Lung Squamous Cell Carcinomas.Cancer Res Commun. 2024 Feb 13;4(2):388-403. doi: 10.1158/2767-9764.CRC-23-0399. Cancer Res Commun. 2024. PMID: 38265267 Free PMC article.
-
New biomarkers for checkpoint inhibitor therapy.ESMO Open. 2020 Sep;5(Suppl 1):e000597. doi: 10.1136/esmoopen-2019-000597. ESMO Open. 2020. PMID: 32933940 Free PMC article. Review.
References
-
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine. 2015;372(21):2018–28. - PubMed
-
- Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. - PubMed
-
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology. 2016;17(11):1497–508. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

