Bag2 Is a Component of a Cytosolic Extraction Machinery That Promotes Membrane Penetration of a Nonenveloped Virus
- PMID: 29769335
- PMCID: PMC6052292
- DOI: 10.1128/JVI.00607-18
Bag2 Is a Component of a Cytosolic Extraction Machinery That Promotes Membrane Penetration of a Nonenveloped Virus
Abstract
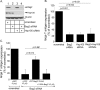
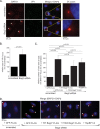
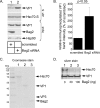
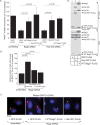
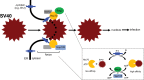
During entry, the nonenveloped polyomavirus (PyV) simian virus 40 (SV40) traffics from the cell surface to the endoplasmic reticulum (ER), where it penetrates the ER membrane to reach the cytosol; the virus is then transported into the nucleus to cause infection. Although a coherent understanding of SV40's host entry is emerging, how the virus is ejected from the ER into the cytosol remains mysterious. Our previous analyses revealed that the cytosolic Hsc70-SGTA-Hsp105 complex binds to SV40 and extracts it from the ER into the cytosol. We now report that the nucleotide exchange factor (NEF) Bag2 stimulates SV40 release from Hsc70, thereby enabling successful virus arrival at the cytosol, which leads to infection. Hsp105, another NEF of Hsc70, displays a function overlapping that of Bag2, underscoring the importance of this release reaction. Our findings identify a new component of an extraction machinery essential during membrane penetration of a nonenveloped virus and provide further mechanistic insights into this process.IMPORTANCE How a nonenveloped virus penetrates a biological membrane to cause infection is a mystery. For the nonenveloped polyomavirus SV40, transport across the ER membrane to reach the cytosol is an essential virus infection step. Here, we identify a novel component of a cytosolic Hsc70-dependent chaperone complex called Bag2 that extracts SV40 from the ER into the cytosol. Bag2 does this by triggering SV40 release from Hsc70, thus ensuring that the virus reaches the cytosol en route for productive infection.
Keywords: endoplasmic reticulum; membrane transport; nonenveloped virus; protein chaperone; simian virus 40.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review.
-
SGTA-Dependent Regulation of Hsc70 Promotes Cytosol Entry of Simian Virus 40 from the Endoplasmic Reticulum.J Virol. 2017 May 26;91(12):e00232-17. doi: 10.1128/JVI.00232-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28356524 Free PMC article.
-
Ubqln4 Facilitates Endoplasmic Reticulum-to-Cytosol Escape of a Nonenveloped Virus during Infection.J Virol. 2020 May 18;94(11):e00103-20. doi: 10.1128/JVI.00103-20. Print 2020 May 18. J Virol. 2020. PMID: 32161173 Free PMC article.
-
A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40.J Virol. 2015 Apr;89(8):4069-79. doi: 10.1128/JVI.03552-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653441 Free PMC article.
-
A bacterial toxin and a nonenveloped virus hijack ER-to-cytosol membrane translocation pathways to cause disease.Crit Rev Biochem Mol Biol. 2015;50(6):477-88. doi: 10.3109/10409238.2015.1085826. Epub 2015 Sep 11. Crit Rev Biochem Mol Biol. 2015. PMID: 26362261 Free PMC article. Review.
Cited by
-
Mechanism and Complex Roles of HSC70 in Viral Infections.Front Microbiol. 2020 Jul 21;11:1577. doi: 10.3389/fmicb.2020.01577. eCollection 2020. Front Microbiol. 2020. PMID: 32849328 Free PMC article. Review.
-
How host ER membrane chaperones and morphogenic proteins support virus infection.J Cell Sci. 2023 Jul 1;136(13):jcs261121. doi: 10.1242/jcs.261121. Epub 2023 Jul 4. J Cell Sci. 2023. PMID: 37401530 Free PMC article.
-
Components of the LINC and NPC complexes coordinately target and translocate a virus into the nucleus to promote infection.PLoS Pathog. 2022 Sep 6;18(9):e1010824. doi: 10.1371/journal.ppat.1010824. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36067270 Free PMC article.
-
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review.
-
Reticulon protects the integrity of the ER membrane during ER escape of large macromolecular protein complexes.J Cell Biol. 2020 Feb 3;219(2):e201908182. doi: 10.1083/jcb.201908182. J Cell Biol. 2020. PMID: 31895406 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

