Synthesis and profiling of a 3-aminopyridin-2-one-based kinase targeted fragment library: Identification of 3-amino-5-(pyridin-4-yl)pyridin-2(1H)-one scaffold for monopolar spindle 1 (MPS1) and Aurora kinases inhibition
- PMID: 29764757
- PMCID: PMC6008489
- DOI: 10.1016/j.bmc.2018.04.033
Synthesis and profiling of a 3-aminopyridin-2-one-based kinase targeted fragment library: Identification of 3-amino-5-(pyridin-4-yl)pyridin-2(1H)-one scaffold for monopolar spindle 1 (MPS1) and Aurora kinases inhibition
Abstract
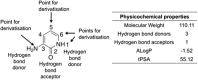
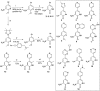
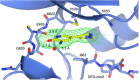
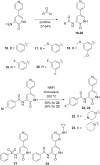
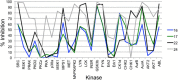
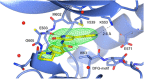
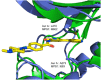
Screening a 3-aminopyridin-2-one based fragment library against a 26-kinase panel representative of the human kinome identified 3-amino-5-(1-methyl-1H-pyrazol-4-yl)pyridin-2(1H)-one (2) and 3-amino-5-(pyridin-4-yl)pyridin-2(1H)-one (3) as ligand efficient inhibitors of the mitotic kinase Monopolar Spindle 1 (MPS1) and the Aurora kinase family. These kinases are well recognised as attractive targets for therapeutic intervention for treating cancer. Elucidation of the binding mode of these fragments and their analogues has been carried out by X-ray crystallography. Structural studies have identified key interactions with a conserved lysine residue and have highlighted potential regions of MPS1 which could be targeted to improve activity and selectivity.
Keywords: 3-Aminopyridin-2-one; Aurora kinase; Fragment compound library; MPS1 kinase.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
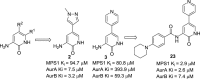
Similar articles
-
A unique hinge binder of extremely selective aminopyridine-based Mps1 (TTK) kinase inhibitors with cellular activity.Bioorg Med Chem. 2015 May 1;23(9):2247-60. doi: 10.1016/j.bmc.2015.02.042. Epub 2015 Mar 4. Bioorg Med Chem. 2015. PMID: 25801152
-
Structural basis of reversine selectivity in inhibiting Mps1 more potently than aurora B kinase.Proteins. 2016 Dec;84(12):1761-1766. doi: 10.1002/prot.25174. Epub 2016 Oct 11. Proteins. 2016. PMID: 27699881
-
Identification and characterisation of 2-aminopyridine inhibitors of checkpoint kinase 2.Bioorg Med Chem. 2010 Jan 15;18(2):707-18. doi: 10.1016/j.bmc.2009.11.058. Epub 2009 Dec 6. Bioorg Med Chem. 2010. PMID: 20022510
-
Molecular design and anticancer activities of small-molecule monopolar spindle 1 inhibitors: A Medicinal chemistry perspective.Eur J Med Chem. 2019 Aug 1;175:247-268. doi: 10.1016/j.ejmech.2019.04.047. Epub 2019 Apr 20. Eur J Med Chem. 2019. PMID: 31121430 Review.
-
Fragment-based approaches to the discovery of kinase inhibitors.Methods Enzymol. 2014;548:69-92. doi: 10.1016/B978-0-12-397918-6.00003-3. Methods Enzymol. 2014. PMID: 25399642 Review.
Cited by
-
Novel Approach to the Synthesis of 3-amino-4-arylpyridin-2(1H)-one Derivatives.Chem Heterocycl Compd (N Y). 2021;57(7-8):764-771. doi: 10.1007/s10593-021-02980-w. Epub 2021 Sep 7. Chem Heterocycl Compd (N Y). 2021. PMID: 34511628 Free PMC article.
References
-
- Zhang J., Yang P.L., Gray N.S. Nat Rev Cancer. 2009;9:28–39. - PubMed
-
- Möbitz H. Biochim Biophys Acta Proteins Proteomics. 2015;1854:1555–1566. - PubMed
-
- Commander H., Whiteside G., Perry C. Drugs. 2011;71:1355–1365. - PubMed
-
- Shaw A.T., Yasothan U., Kirkpatrick P. Nat Rev Drug Discov. 2011;10:897–898. - PubMed
-
- Dolgin E. Nat Rev Drug Discov. 2011;10:717–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

