Effects of gel volume on pharmacokinetics for vaginal and rectal applications of combination DuoGel-IQB4012, a dual chamber-dual drug HIV microbicide gel, in pigtailed macaques
- PMID: 29761350
- PMCID: PMC6711467
- DOI: 10.1007/s13346-018-0538-0
Effects of gel volume on pharmacokinetics for vaginal and rectal applications of combination DuoGel-IQB4012, a dual chamber-dual drug HIV microbicide gel, in pigtailed macaques
Abstract
This study evaluated effects of differing gel volumes on pharmacokinetics (PK). IQB4012, a gel containing the non-nucleoside reverse transcriptase inhibitor IQP-0528 and tenofovir (TFV), was applied to the pigtailed macaque vagina and rectum. Vaginal gel volumes (1% loading of both drugs) were 0.5 or 1.5 ml; following wash-out, 1 or 4 ml of gel were then applied rectally. Blood, vaginal, and rectal fluids were collected at 0, 2, 4, and 24 h. Vaginal and rectal tissue biopsies were collected at 4 and 24 h. There were no statistically significant differences in concentrations for either drug between gel volumes within compartments at matched time points. After vaginal gel application, median IQP-0528 concentrations were ~ 104-105 ng/g, 105-106 ng/ml, and 103-105 ng/ml in vaginal tissues, vaginal fluids, and rectal fluids, respectively (over 24 h). Median vaginal TFV concentrations were 1-2 logs lower than IQP-0528 levels at matched time points. After rectal gel application, median IQP-0528 and TFV concentrations in rectal fluids were ~ 103-105 ng/ml and ~ 102-103 ng/ml, respectively. Concentrations of both drugs sampled in rectal tissues were low (~ 101-103 ng/g). For 1 ml gel, half of sampled rectal tissues had undetectable concentrations of either drug, and over half of sampled rectal fluids had undetectable TFV concentrations. These results indicate differences in drug delivery between the vaginal and rectal compartments, and that smaller vaginal gel volumes may not significantly compromise microbicide PK and prophylactic potential. However, effects of rectal gel volume on PK for both drugs were less definitive.
Keywords: HIV prevention; IQP0528; Macaque; Pharmacokinetics; PrEP; Rectal gel; Vaginal gel.
Conflict of interest statement
Figures
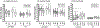
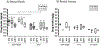
Similar articles
-
Pharmacokinetics and Pharmacodynamics of Tenofovir Reduced-Glycerin 1% Gel in the Rectal and Vaginal Compartments in Women: A Cross-Compartmental Study With Directly Observed Dosing.J Acquir Immune Defic Syndr. 2018 Jun 1;78(2):175-182. doi: 10.1097/QAI.0000000000001655. J Acquir Immune Defic Syndr. 2018. PMID: 29767639 Free PMC article. Clinical Trial.
-
Pharmacokinetic and Pharmacodynamic Evaluation following Vaginal Application of IQB3002, a Dual-Chamber Microbicide Gel Containing the Nonnucleoside Reverse Transcriptase Inhibitor IQP-0528 in Rhesus Macaques.Antimicrob Agents Chemother. 2015 Dec 14;60(3):1393-400. doi: 10.1128/AAC.02201-15. Antimicrob Agents Chemother. 2015. PMID: 26666935 Free PMC article.
-
Differences in Local and Systemic TFV PK Among Premenopausal Versus Postmenopausal Women Exposed to TFV 1% Vaginal Gel.J Acquir Immune Defic Syndr. 2018 May 1;78(1):82-92. doi: 10.1097/QAI.0000000000001648. J Acquir Immune Defic Syndr. 2018. PMID: 29424790 Free PMC article.
-
The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women.Curr HIV/AIDS Rep. 2017 Oct;14(5):153-160. doi: 10.1007/s11904-017-0362-z. Curr HIV/AIDS Rep. 2017. PMID: 28812207 Free PMC article. Review.
-
Vaginal drug distribution modeling.Adv Drug Deliv Rev. 2015 Sep 15;92:2-13. doi: 10.1016/j.addr.2015.04.017. Epub 2015 Apr 28. Adv Drug Deliv Rev. 2015. PMID: 25933938 Free PMC article. Review.
Cited by
-
Deducing Mucosal Pharmacokinetics and Pharmacodynamics of the Anti-HIV Molecule Tenofovir from Measurements in Blood.Sci Rep. 2019 Jan 14;9(1):82. doi: 10.1038/s41598-018-36004-z. Sci Rep. 2019. PMID: 30643165 Free PMC article.
-
Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse.Pharmaceutics. 2019 May 13;11(5):232. doi: 10.3390/pharmaceutics11050232. Pharmaceutics. 2019. PMID: 31086015 Free PMC article.
References
-
- FDA approves first drug for reducing the risk of sexually acquired HIV infection [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm2012.
-
- Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

