How to prove the existence of metabolons?
- PMID: 29755303
- PMCID: PMC5932110
- DOI: 10.1007/s11101-017-9509-1
How to prove the existence of metabolons?
Abstract
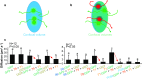
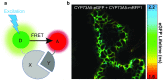
Sequential enzymes in biosynthetic pathways are organized in metabolons. It is challenging to provide experimental evidence for the existence of metabolons as biosynthetic pathways are composed of highly dynamic protein-protein interactions. Many different methods are being applied, each with strengths and weaknesses. We will present and evaluate several techniques that have been applied in providing evidence for the orchestration of the biosynthetic pathways of cyanogenic glucosides and glucosinolates in metabolons. These evolutionarily related pathways have ER-localized cytochromes P450 that are proposed to function as anchoring site for assembly of the enzymes into metabolons. Additionally, we have included commonly used techniques, even though they have not been used (yet) on these two pathways. In the review, special attention will be given to less-exploited fluorescence-based methods such as FCS and FLIM. Ultimately, understanding the orchestration of biosynthetic pathways may contribute to successful engineering in heterologous hosts.
Keywords: Fluorescence correlation spectroscopy; Fluorescence lifetime imaging microscopy; Fluorescence-based protein–protein interaction; Yeast-2-hybrid screen.
Figures


Similar articles
-
Synthetic metabolons for metabolic engineering.J Exp Bot. 2014 May;65(8):1947-54. doi: 10.1093/jxb/eru050. Epub 2014 Mar 3. J Exp Bot. 2014. PMID: 24591054 Review.
-
Formation of Flavonoid Metabolons: Functional Significance of Protein-Protein Interactions and Impact on Flavonoid Chemodiversity.Front Plant Sci. 2019 Jul 9;10:821. doi: 10.3389/fpls.2019.00821. eCollection 2019. Front Plant Sci. 2019. PMID: 31338097 Free PMC article. Review.
-
Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum.Science. 2016 Nov 18;354(6314):890-893. doi: 10.1126/science.aag2347. Science. 2016. PMID: 27856908
-
Identification of protein-protein interactions of isoflavonoid biosynthetic enzymes with 2-hydroxyisoflavanone synthase in soybean (Glycine max (L.) Merr.).Biochem Biophys Res Commun. 2016 Jan 15;469(3):546-51. doi: 10.1016/j.bbrc.2015.12.038. Epub 2015 Dec 13. Biochem Biophys Res Commun. 2016. PMID: 26694697
-
Plasticity of specialized metabolism as mediated by dynamic metabolons.Trends Plant Sci. 2015 Jan;20(1):20-32. doi: 10.1016/j.tplants.2014.11.002. Epub 2014 Nov 27. Trends Plant Sci. 2015. PMID: 25435320 Review.
Cited by
-
Enzyme Complexes of Ptr4CL and PtrHCT Modulate Co-enzyme A Ligation of Hydroxycinnamic Acids for Monolignol Biosynthesis in Populus trichocarpa.Front Plant Sci. 2021 Oct 6;12:727932. doi: 10.3389/fpls.2021.727932. eCollection 2021. Front Plant Sci. 2021. PMID: 34691108 Free PMC article.
-
A roadmap of plant membrane transporters in arbuscular mycorrhizal and legume-rhizobium symbioses.Plant Physiol. 2021 Dec 4;187(4):2071-2091. doi: 10.1093/plphys/kiab280. Plant Physiol. 2021. PMID: 34618047 Free PMC article. Review.
-
The Phenylpropanoid Case - It Is Transport That Matters.Front Plant Sci. 2018 Nov 1;9:1610. doi: 10.3389/fpls.2018.01610. eCollection 2018. Front Plant Sci. 2018. PMID: 30443262 Free PMC article.
-
The Purinosome: A Case Study for a Mammalian Metabolon.Annu Rev Biochem. 2022 Jun 21;91:89-106. doi: 10.1146/annurev-biochem-032620-105728. Epub 2022 Mar 23. Annu Rev Biochem. 2022. PMID: 35320684 Free PMC article. Review.
-
Dynamic metabolic solutions to the sessile life style of plants.Nat Prod Rep. 2018 Nov 14;35(11):1140-1155. doi: 10.1039/c8np00037a. Nat Prod Rep. 2018. PMID: 30324199 Free PMC article. Review.
References
-
- Aker J, Hesselink R, Engel R, et al. In vivo hexamerization and characterization of the Arabidopsis AAA ATPase CDC48A complex using forster resonance energy transfer-fluorescence lifetime imaging microscopy and fluorescence correlation spectroscopy. Plant Physiol. 2007;145(2):339–350. - PMC - PubMed
-
- An S, Kumar R, Sheets ED, et al. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320(5872):103–106. - PubMed
-
- Andersen TG (2012) Novel molecular tools for crop development and synthesis of natural products—understanding glucosinolate based defenses in cruciferous plants. Ph.D. dissertation, University of Copenhagen
-
- Andrès C, Agne B, Kessler F. Preparation of multiprotein complexes from Arabidopsis chloroplasts using tandem affinity purification. Methods Mol Biol. 2011;775:31–49. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
