Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media
- PMID: 29751821
- PMCID: PMC5948766
- DOI: 10.1186/s13287-018-0886-1
Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media
Abstract
Background: The stromal vascular fraction (SVF) derived from adipose tissue contains adipose-derived stromal/stem cells (ASC) and can be used for regenerative applications. Thus, a validated protocol for SVF isolation, freezing, and thawing is required to manage product administration. To comply with Good Manufacturing Practice (GMP), fetal bovine serum (FBS), used to expand ASC in vitro, could be replaced by growth factors from platelet concentrates.
Methods: Throughout each protocol, GMP-compliant reagents and devices were used. SVF cells were isolated from lipoaspirates by a standardized enzymatic protocol. Cells were cryopreserved in solutions containing different albumin or serum and dimethylsulfoxide (DMSO) concentrations. Before and after cryopreservation, we analyzed: cell viability (by Trypan blue); immunophenotype (by flow cytometry); colony-forming unit-fibroblast (CFU-F) formation; and differentiation potential. ASC, seeded at different densities, were expanded in presence of 10% FBS or 5% supernatant rich in growth factors (SRGF) from platelets. The differentiation potential and cell transformation grade were tested in expanded ASC.
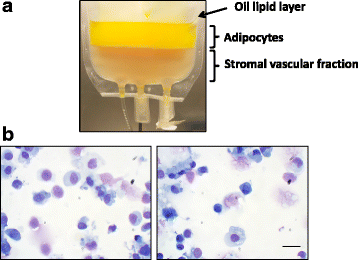
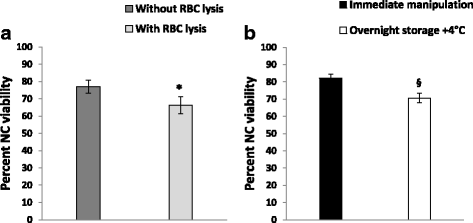
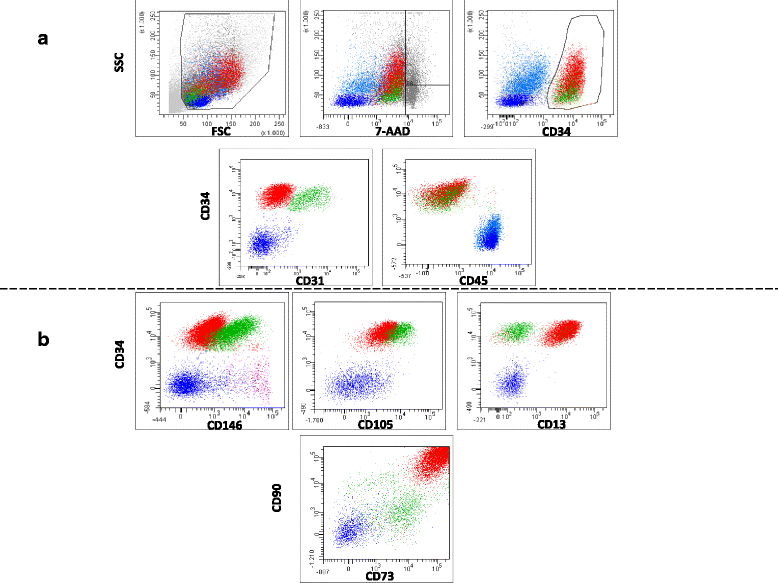
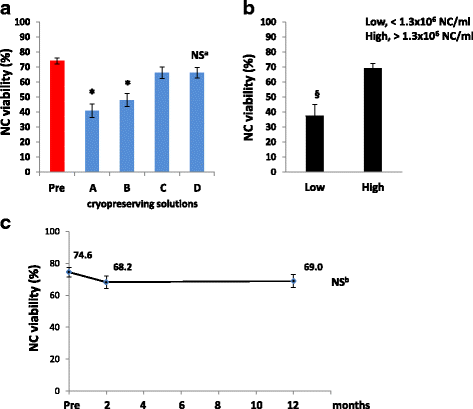
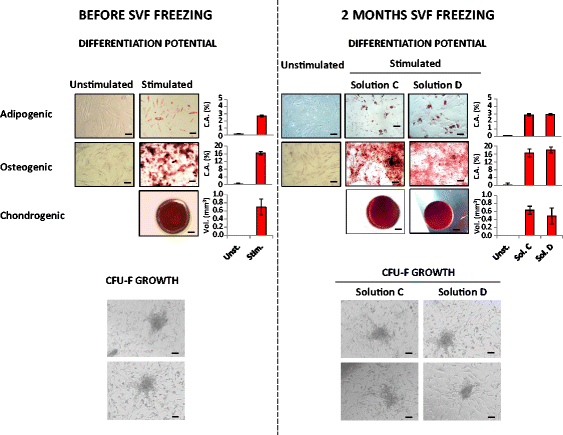
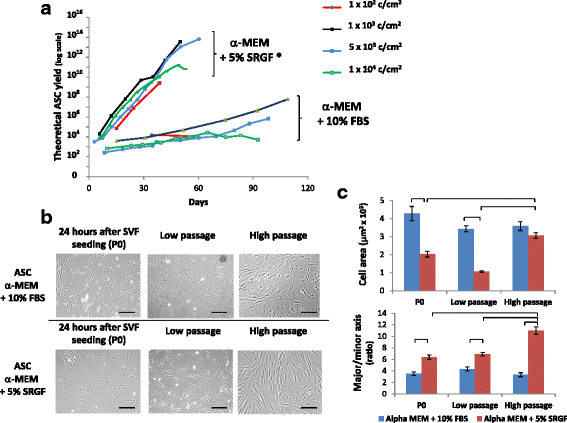
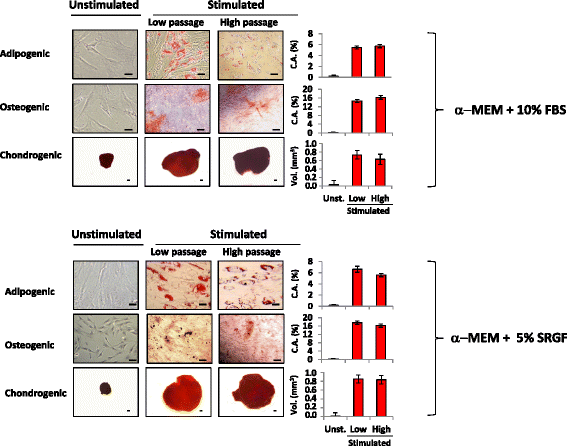
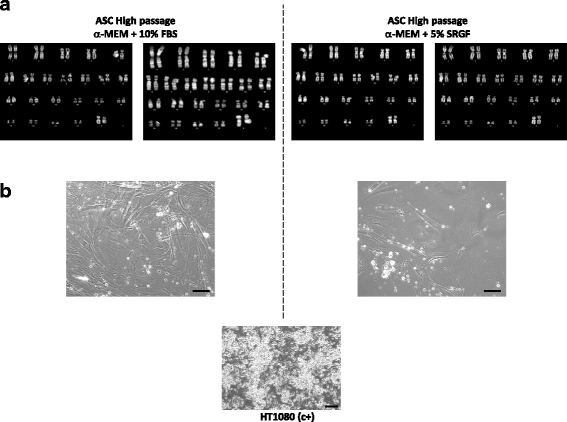
Results: We demonstrated that SVF can be obtained with a consistent yield (about 185 × 103 cells/ml lipoaspirate) and viability (about 82%). Lipoaspirate manipulation after overnight storage at +4 °C reduced cell viability (-11.6%). The relative abundance of ASC (CD34+CD45-CD31-) and endothelial precursors (CD34+CD45-CD31+) in the SVF product was about 59% and 42%, respectively. A period of 2 months cryostorage in autologous serum with added DMSO minimally affected post-thaw SVF cell viability as well as clonogenic and differentiation potentials. Viability was negatively affected when SVF was frozen at a cell concentration below 1.3 × 106 cells/ml. Cell viability was not significantly affected after a freezing period of 1 year. Independent of seeding density, ASC cultured in 5% SRGF exhibited higher growth rates when compared with 10% FBS. ASC expanded in both media showed unaltered identity (by flow cytometry) and were exempt from genetic lesions. Both 5% SRGF- and 10% FBS-expanded ASC efficiently differentiated to adipocytes, osteocytes, and chondrocytes.
Conclusions: This paper reports a GMP-compliant approach for freezing SVF cells isolated from adipose tissue by a standardized protocol. Moreover, an ASC expansion method in controlled culture conditions and without involvement of animal-derived additives was reported.
Keywords: Adipose stem/stromal stem cells; Adipose tissue; Advanced therapy medicinal product; Anchorage independent growth; CFU-F; Cell morphology; Cell viability; Differentiation potential; Freezing protocol; Good manufacturing practice; Growth rate; Immunophenotype characterization; Karyotype; Stromal vascular fraction.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the CRO Aviano National Cancer Institute (protocol number: CRO-2016-30), and it was performed in accordance with the Declaration of Helsinki (2004). Signed informed consent was collected from patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Non-toxic freezing media to retain the stem cell reserves in adipose tissues.Cryobiology. 2020 Oct;96:137-144. doi: 10.1016/j.cryobiol.2020.07.005. Epub 2020 Jul 17. Cryobiology. 2020. PMID: 32687840
-
Mechanical process prior to cryopreservation of lipoaspirates maintains extracellular matrix integrity and cell viability: evaluation of the retention and regenerative potential of cryopreserved fat-derived product after fat grafting.Stem Cell Res Ther. 2019 Sep 23;10(1):283. doi: 10.1186/s13287-019-1395-6. Stem Cell Res Ther. 2019. PMID: 31547884 Free PMC article.
-
Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium.J Tissue Eng Regen Med. 2010 Mar;4(3):224-32. doi: 10.1002/term.232. J Tissue Eng Regen Med. 2010. PMID: 19967746 Free PMC article.
-
Cryopreserved Adipose Tissue-Derived Stromal/Stem Cells: Potential for Applications in Clinic and Therapy.Adv Exp Med Biol. 2016;951:137-146. doi: 10.1007/978-3-319-45457-3_11. Adv Exp Med Biol. 2016. PMID: 27837560 Review.
-
Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy.Crit Rev Eukaryot Gene Expr. 2015;25(2):145-52. doi: 10.1615/critreveukaryotgeneexpr.2015013057. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080608 Review.
Cited by
-
Controversies regarding transplantation of mesenchymal stem cells.World J Transplant. 2024 Jun 18;14(2):90554. doi: 10.5500/wjt.v14.i2.90554. World J Transplant. 2024. PMID: 38947963 Free PMC article. Review.
-
Cryostorage of Mesenchymal Stem Cells and Biomedical Cell-Based Products.Cells. 2022 Aug 29;11(17):2691. doi: 10.3390/cells11172691. Cells. 2022. PMID: 36078098 Free PMC article. Review.
-
Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction From Adipose Tissue: Preliminary Results.Front Cell Dev Biol. 2019 Jun 7;7:88. doi: 10.3389/fcell.2019.00088. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31231649 Free PMC article.
-
Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview.Int J Mol Sci. 2018 Jun 28;19(7):1897. doi: 10.3390/ijms19071897. Int J Mol Sci. 2018. PMID: 29958391 Free PMC article. Review.
-
Characterisation of Novel Angiogenic and Potent Anti-Inflammatory Effects of Micro-Fragmented Adipose Tissue.Int J Mol Sci. 2021 Mar 23;22(6):3271. doi: 10.3390/ijms22063271. Int J Mol Sci. 2021. PMID: 33806897 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

