The aging lung: tissue telomere shortening in health and disease
- PMID: 29751799
- PMCID: PMC5948770
- DOI: 10.1186/s12931-018-0794-z
The aging lung: tissue telomere shortening in health and disease
Abstract
Background: Telomere shortening has been associated with several lung diseases. However, telomere length is generally measured in peripheral blood leucocytes rather than in lung tissue, where disease occurs. Consequently, telomere dynamics have not been established for the normal human lung nor for diseased lung tissue. We hypothesized an age- and disease-dependent shortening of lung tissue telomeres.
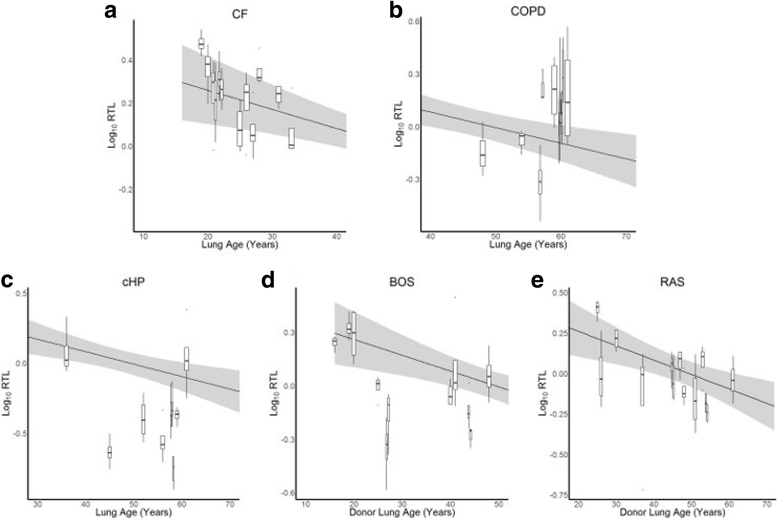
Methods: At time of (re-)transplantation or autopsy, 70 explant lungs were collected: from unused donors (normal, n = 13) and patients with cystic fibrosis (CF, n = 12), chronic obstructive pulmonary disease (COPD, n = 11), chronic hypersensitivity pneumonitis (cHP, n = 9), bronchiolitis obliterans syndrome (BOS) after prior transplantation (n = 11) and restrictive allograft syndrome (RAS) after prior transplantation (n = 14). Lungs were inflated, frozen and then scanned using CT. Four tissue cores from distinct lung regions were sampled for analysis. Disease severity was evaluated using CT and micro CT imaging. DNA was extracted from the samples and average relative telomere length (RTL) was determined using real-time qPCR.
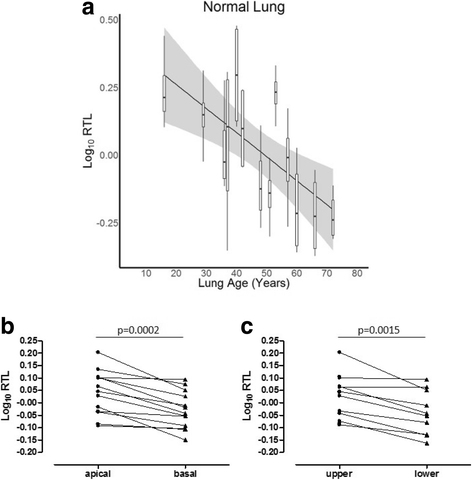
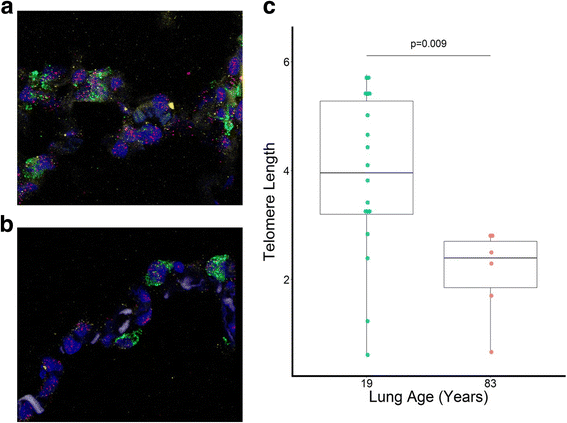
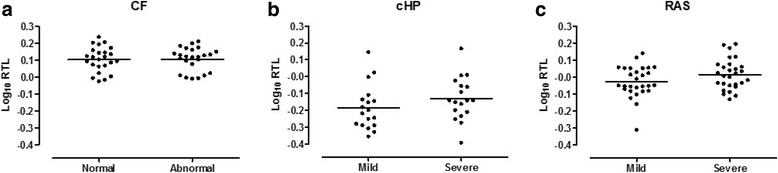
Results: The normal lungs showed a decrease in RTL with age (p < 0.0001). Of the diseased lungs, only BOS and RAS showed significant RTL decrease with increasing lung age (p = 0.0220 and p = 0.0272 respectively). Furthermore, we found that RTL showed considerable variability between samples within both normal and diseased lungs. cHP, BOS and RAS lungs had significant shorter RTL in comparison with normal lungs, after adjustment for lung age, sex and BMI (p < 0.0001, p = 0.0051 and p = 0.0301 respectively). When investigating the relation between RTL and regional disease severity in CF, cHP and RAS, no association was found.
Conclusion: These results show a progressive decline in telomere length with age in normal, BOS and RAS lungs. cHP, BOS and RAS lungs demonstrated shorter RTL compared to normal lungs. Lung tissue RTL does not associate with regional disease severity within the lung. Therefore, tissue RTL does not seem to fully reflect peripheral blood telomere length.
Keywords: BOS; Cellular senescence; Chronic hypersensitivity pneumonitis; Chronic lung allograft dysfunction; Chronic obstructive pulmonary disease; Cystic fibrosis; RAS; Telomere length.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the local Ethics Committee (Medical Ethics Board of University Hospitals Leuven, Belgium; ML6385). According to Belgian law, organs from prospective donors, which are of insufficient quality for LTx and have been conclusively declined by the transplant surgeon, can be used for research purposes. All LTx patients gave written informed consent to use their lungs for research purposes.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Comparison of the relative telomere length measured in leukocytes and eleven different human tissues.Physiol Res. 2014;63(Suppl 3):S343-50. doi: 10.33549/physiolres.932856. Physiol Res. 2014. PMID: 25428739
-
Intragraft donor-specific anti-HLA antibodies in phenotypes of chronic lung allograft dysfunction.Eur Respir J. 2019 Nov 7;54(5):1900847. doi: 10.1183/13993003.00847-2019. Print 2019 Nov. Eur Respir J. 2019. PMID: 31439680
-
Connective Tissue Growth Factor Is Overexpressed in Explant Lung Tissue and Broncho-Alveolar Lavage in Transplant-Related Pulmonary Fibrosis.Front Immunol. 2021 May 25;12:661761. doi: 10.3389/fimmu.2021.661761. eCollection 2021. Front Immunol. 2021. PMID: 34122421 Free PMC article.
-
Bronchiolitis obliterans syndrome and restrictive allograft syndrome after lung transplantation: why are there two distinct forms of chronic lung allograft dysfunction?Ann Transl Med. 2020 Mar;8(6):418. doi: 10.21037/atm.2020.02.159. Ann Transl Med. 2020. PMID: 32355862 Free PMC article. Review.
-
Telomere Shortening and Its Association with Cell Dysfunction in Lung Diseases.Int J Mol Sci. 2021 Dec 31;23(1):425. doi: 10.3390/ijms23010425. Int J Mol Sci. 2021. PMID: 35008850 Free PMC article. Review.
Cited by
-
Remodeling of the Aged and Emphysematous Lungs: Roles of Microenvironmental Cues.Compr Physiol. 2022 Jun 29;12(3):3559-3574. doi: 10.1002/cphy.c210033. Compr Physiol. 2022. PMID: 35766835 Free PMC article.
-
Towards the Essence of Progressiveness: Bringing Progressive Fibrosing Interstitial Lung Disease (PF-ILD) to the Next Stage.J Clin Med. 2020 Jun 3;9(6):1722. doi: 10.3390/jcm9061722. J Clin Med. 2020. PMID: 32503224 Free PMC article.
-
Cystic Fibrosis Lung Disease in the Aging Population.Front Pharmacol. 2021 Apr 15;12:601438. doi: 10.3389/fphar.2021.601438. eCollection 2021. Front Pharmacol. 2021. PMID: 33935699 Free PMC article. Review.
-
The aging lung: Physiology, disease, and immunity.Cell. 2021 Apr 15;184(8):1990-2019. doi: 10.1016/j.cell.2021.03.005. Epub 2021 Apr 2. Cell. 2021. PMID: 33811810 Free PMC article. Review.
-
Telomeres in Interstitial Lung Disease: The Short and the Long of It.Ann Am Thorac Soc. 2019 Feb;16(2):175-181. doi: 10.1513/AnnalsATS.201808-508CME. Ann Am Thorac Soc. 2019. PMID: 30540921 Free PMC article. Review.
References
-
- Brandenberger C, Mühlfeld C. Mechanisms of lung aging. Cell Tissue Res. 2016;14:1–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

