A single amino acid substitution in the Bombyx-specific mucin-like membrane protein causes resistance to Bombyx mori densovirus
- PMID: 29743532
- PMCID: PMC5943349
- DOI: 10.1038/s41598-018-25388-7
A single amino acid substitution in the Bombyx-specific mucin-like membrane protein causes resistance to Bombyx mori densovirus
Abstract
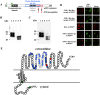
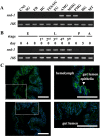
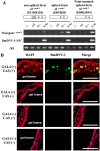
Bombyx mori densovirus type 1 (BmDV) is a pathogen that causes flacherie disease in the silkworm. The absolute nonsusceptibility to BmDV among certain silkworm strains is determined independently by two genes, nsd-1 and Nid-1. However, neither of these genes has been molecularly identified to date. Here, we isolated the nsd-1 gene by positional cloning and characterized the properties of its product, NSD-1. Sequence and biochemical analyses revealed that this gene encodes a Bombyx-specific mucin-like glycoprotein with a single transmembrane domain. The NSD-1 protein was specifically expressed in the larval midgut epithelium, the known infection site of BmDV. Sequence analysis of the nsd-1 gene from 13 resistant and 12 susceptible strains suggested that a specific arginine residue in the extracellular tail of the NSD-1 protein was common among susceptible strains. Germline transformation of the susceptible-type nsd-1 (with a single nucleotide substitution) conferred partial susceptibility to resistant larvae, indicating that the + nsd-1 gene is required for the susceptibility of B. mori larvae to BmDV and the susceptibility is solely a result of the substitution of a single amino acid with arginine. Taken together, our results provide striking evidence that a novel membrane-bound mucin-like protein functions as a cell-surface receptor for a densovirus.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori).Antioxidants (Basel). 2021 Mar 27;10(4):522. doi: 10.3390/antiox10040522. Antioxidants (Basel). 2021. PMID: 33801623 Free PMC article. Review.
-
Resistance mechanism of Nid-1, a dominant non-susceptibility gene, against Bombyx mori densovirus 1 infection.Virus Res. 2022 Sep;318:198849. doi: 10.1016/j.virusres.2022.198849. Epub 2022 Jun 9. Virus Res. 2022. PMID: 35691422
-
Gene expression and localization analysis of Bombyx mori bidensovirus and its putative receptor in B. mori midgut.J Invertebr Pathol. 2016 May;136:50-6. doi: 10.1016/j.jip.2016.03.005. Epub 2016 Mar 4. J Invertebr Pathol. 2016. PMID: 26953258
-
Decrease in the expression level of the gene encoding the putative Bombyx mori bidensovirus receptor during virus infection.Arch Virol. 2018 Dec;163(12):3327-3338. doi: 10.1007/s00705-018-4017-1. Epub 2018 Sep 15. Arch Virol. 2018. PMID: 30220036
-
Non-susceptibility genes to Bombyx densovirus type 1, Nid-1 and nsd-1, affect distinct steps of the viral infection pathway.J Invertebr Pathol. 2010 Jan;103(1):79-81. doi: 10.1016/j.jip.2009.10.006. Epub 2009 Oct 27. J Invertebr Pathol. 2010. PMID: 19836396
Cited by
-
Endogenous Viral Element-Derived Piwi-Interacting RNAs (piRNAs) Are Not Required for Production of Ping-Pong-Dependent piRNAs from Diaphorina citri Densovirus.mBio. 2020 Sep 29;11(5):e02209-20. doi: 10.1128/mBio.02209-20. mBio. 2020. PMID: 32994324 Free PMC article.
-
Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori).Antioxidants (Basel). 2021 Mar 27;10(4):522. doi: 10.3390/antiox10040522. Antioxidants (Basel). 2021. PMID: 33801623 Free PMC article. Review.
-
Interaction of a Densovirus with Glycans of the Peritrophic Matrix Mediates Oral Infection of the Lepidopteran Pest Spodoptera frugiperda.Viruses. 2019 Sep 17;11(9):870. doi: 10.3390/v11090870. Viruses. 2019. PMID: 31533310 Free PMC article.
-
A Single SNP Turns a Social Honey Bee (Apis mellifera) Worker into a Selfish Parasite.Mol Biol Evol. 2019 Mar 1;36(3):516-526. doi: 10.1093/molbev/msy232. Mol Biol Evol. 2019. PMID: 30624681 Free PMC article.
References
-
- Kawase, S. & Kurstak, E. In Viruses of Invertebrates (ed Kurstak E) 315–343 (Marcel Dekker, New York, 1991).
-
- Tijssen P, Bergoin M. Densonucleosis viruses constitute an increasingly diversified subfamily among the parvoviruses. Semin. Virol. 1995;6:347–355. doi: 10.1006/smvy.1995.0041. - DOI
-
- Watanabe H, Maeda S, Matsui M, Shimizu T. Histopathology of the midgut epithelium of the silkworm, Bombyx mori, infected with a newly-isolated virus from the flacherie-diseased larvae. J. Seric. Sci. Jpn. 1976;45:29–34.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

