Breadth and Functionality of Varicella-Zoster Virus Glycoprotein-Specific Antibodies Identified after Zostavax Vaccination in Humans
- PMID: 29743372
- PMCID: PMC6026762
- DOI: 10.1128/JVI.00269-18
Breadth and Functionality of Varicella-Zoster Virus Glycoprotein-Specific Antibodies Identified after Zostavax Vaccination in Humans
Abstract
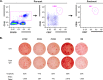
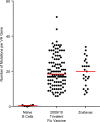
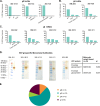
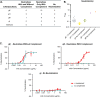
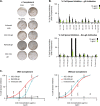
Herpes zoster (HZ) (shingles) is the clinical manifestation of varicella-zoster virus (VZV) reactivation. HZ typically develops as people age, due to decreased cell-mediated immunity. However, the importance of antibodies for immunity against HZ prevention remains to be understood. The goal of this study was to examine the breadth and functionality of VZV-specific antibodies after vaccination with a live attenuated HZ vaccine (Zostavax). Direct enumeration of VZV-specific antibody-secreting cells (ASCs) via enzyme-linked immunosorbent spot assay (ELISPOT assay) showed that Zostavax can induce both IgG and IgA ASCs 7 days after vaccination but not IgM ASCs. The VZV-specific ASCs range from 33 to 55% of the total IgG ASCs. Twenty-five human VZV-specific monoclonal antibodies (MAbs) were cloned and characterized from single-cell-sorted ASCs of five subjects (>60 years old) who received Zostavax. These MAbs had an average of ∼20 somatic hypermutations per VH gene, similar to those seen after seasonal influenza vaccination. Fifteen of the 25 MAbs were gE specific, whereas the remaining MAbs were gB, gH, or gI specific. The most potent neutralizing antibodies were gH specific and were also able to inhibit cell-to-cell spread of the virus in vitro Most gE-specific MAbs were able to neutralize VZV, but they required the presence of complement and were unable to block cell-to-cell spread. These data indicate that Zostavax induces a memory B cell recall response characterized by anti-gE > anti-gI > anti-gB > anti-gH antibodies. While antibodies to gH could be involved in limiting the spread of VZV upon reactivation, the contribution of anti-gE antibodies toward protective immunity after Zostavax needs further evaluation.IMPORTANCE Varicella-zoster virus (VZV) is the causative agent of chickenpox and shingles. Following infection with VZV, the virus becomes latent and resides in nerve cells. Age-related declines in immunity/immunosuppression can result in reactivation of this latent virus, causing shingles. It has been shown that waning T cell immunity correlates with an increased incidence of VZV reactivation. Interestingly, serum with high levels of VZV-specific antibodies (VariZIG; IV immunoglobulin) has been administered to high-risk populations, e.g., immunocompromised children, newborns, and pregnant women, after exposure to VZV and has shown some protection against chickenpox. However, the relative contribution of antibodies against individual surface glycoproteins toward protection from shingles in elderly/immunocompromised individuals has not been established. Here, we examined the breadth and functionality of VZV-specific antibodies after vaccination with the live attenuated VZV vaccine Zostavax in humans. This study will add to our understanding of the role of antibodies in protection against shingles.
Keywords: B cell; VZV; VZV glycoprotein; Zostavax; antibody; cloning; herpes zoster; immunology; shingles.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Comparative Antibody Responses to the Live-Attenuated and Recombinant Herpes Zoster Vaccines.J Virol. 2021 May 24;95(12):e00240-21. doi: 10.1128/JVI.00240-21. Print 2021 May 24. J Virol. 2021. PMID: 33762414 Free PMC article. Clinical Trial.
-
Identification and Characterization of CD4+ T Cell Epitopes after Shingrix Vaccination.J Virol. 2020 Nov 23;94(24):e01641-20. doi: 10.1128/JVI.01641-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999027 Free PMC article.
-
Zoster vaccine (Zostavax): a review of its use in preventing herpes zoster and postherpetic neuralgia in older adults.Drugs Aging. 2010 Feb 1;27(2):159-76. doi: 10.2165/10489140-000000000-00000. Drugs Aging. 2010. PMID: 20104941 Review.
-
The inactivated herpes zoster vaccine HZ/su induces a varicella zoster virus specific cellular and humoral immune response in patients on dialysis.EBioMedicine. 2024 Oct;108:105335. doi: 10.1016/j.ebiom.2024.105335. Epub 2024 Sep 11. EBioMedicine. 2024. PMID: 39265505 Free PMC article.
-
Understanding the immunology of the Zostavax shingles vaccine.Curr Opin Immunol. 2019 Aug;59:25-30. doi: 10.1016/j.coi.2019.02.005. Epub 2019 Apr 7. Curr Opin Immunol. 2019. PMID: 30970291 Review.
Cited by
-
Structures of the Varicella Zoster Virus Glycoprotein E and Epitope Mapping of Vaccine-Elicited Antibodies.Vaccines (Basel). 2024 Sep 27;12(10):1111. doi: 10.3390/vaccines12101111. Vaccines (Basel). 2024. PMID: 39460278 Free PMC article.
-
Potent and long-lasting humoral and cellular immunity against varicella zoster virus induced by mRNA-LNP vaccine.NPJ Vaccines. 2024 Apr 4;9(1):72. doi: 10.1038/s41541-024-00865-5. NPJ Vaccines. 2024. PMID: 38575581 Free PMC article.
-
Selective retention of virus-specific tissue-resident T cells in healed skin after recovery from herpes zoster.Nat Commun. 2022 Nov 15;13(1):6957. doi: 10.1038/s41467-022-34698-4. Nat Commun. 2022. PMID: 36376285 Free PMC article.
-
Comparative Antibody Responses to the Live-Attenuated and Recombinant Herpes Zoster Vaccines.J Virol. 2021 May 24;95(12):e00240-21. doi: 10.1128/JVI.00240-21. Print 2021 May 24. J Virol. 2021. PMID: 33762414 Free PMC article. Clinical Trial.
-
The next frontier in vaccine design: blending immune correlates of protection into rational vaccine design.Curr Opin Immunol. 2022 Oct;78:102234. doi: 10.1016/j.coi.2022.102234. Epub 2022 Aug 13. Curr Opin Immunol. 2022. PMID: 35973352 Free PMC article. Review.
References
-
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL, et al. . 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352:2271–2284. doi:10.1056/NEJMoa051016. - DOI - PubMed
-
- Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. 2013. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis 208:1953–1961. doi:10.1093/infdis/jit365. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

