Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus
- PMID: 29743371
- PMCID: PMC6052307
- DOI: 10.1128/JVI.00572-18
Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus
Abstract
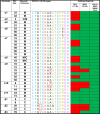
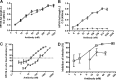
To address how L2-specific antibodies prevent human papillomavirus (HPV) infection of the genital tract, we generated neutralizing monoclonal antibodies (MAbs) WW1, a rat IgG2a that binds L2 residues 17 to 36 (like mouse MAb RG1), and JWW3, a mouse IgG2b derivative of Mab24 specific for L2 residues 58 to 64. By Western blotting, WW1 recognized L2 of 29/34 HPV genotypes tested, compared to only 13/34 for RG1 and 25/34 for JWW3. WW1 IgG and F(ab')2 bound HPV16 pseudovirions similarly; however, whole IgG provided better protection against HPV vaginal challenge. Passive transfer of WW1 IgG was similarly protective in wild-type and neonatal Fc receptor (FcRn)-deficient mice, suggesting that protection by WW1 IgG is not mediated by FcRn-dependent transcytosis. Rather, local epithelial disruption, required for genital infection and induced by either brushing or nonoxynol-9 treatment, released serum IgG in the genital tract, suggesting Fc-independent exudation. Depletion of neutrophils and macrophages reduced protection of mice upon passive transfer of whole WW1 or JWW3 IgGs. Similarly, IgG-mediated protection by L2 MAbs WW1, JWW3, and RG1 was reduced in Fc receptor knockout compared to wild-type mice. However, levels of in vitro neutralization by WW1 IgG were similar in TRIM21 knockout and wild-type cells, indicating that Fc does not contribute to antibody-dependent intracellular neutralization (ADIN). In conclusion, the Fc domain of L2-specific IgGs is not active for ADIN, but it opsonizes bound extracellular pseudovirions for phagocytes in protecting mice from intravaginal HPV challenge. Systemically administered neutralizing IgG can access the site of infection in an abrasion via exudation without the need for FcRn-mediated transcytosis.IMPORTANCE At least 15 alpha HPV types are causative agents for 5% of all cancers worldwide, and beta types have been implicated in nonmelanoma skin cancer, whereas others produce benign papillomas, such as genital warts, associated with considerable morbidity and health systems costs. Vaccines targeting the minor capsid protein L2 have the potential to provide broad-spectrum immunity against medically relevant HPVs of divergent genera via the induction of broadly cross-neutralizing serum IgG. Here we examine the mechanisms by which L2-specific serum IgG reaches the viral inoculum in the genital tract to effect protection. Abrasion of the vaginal epithelium allows the virus to access and infect basal keratinocytes, and our findings suggest that this also permits the local exudation of neutralizing IgG and vaccine-induced sterilizing immunity. We also demonstrate the importance of Fc-mediated phagocytosis of L2 antibody-virion complexes for humoral immunity, a protective mechanism that is not detected by current in vitro neutralization assays.
Keywords: Fc; FcRn; L2 protein; TRIM21; antibody-mediated protection; cross-protection; human papillomavirus; humoral immunity; monoclonal antibody; neutralizing antibodies.
Copyright © 2018 American Society for Microbiology.
Figures



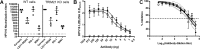
Similar articles
-
Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection.Proc Natl Acad Sci U S A. 2011 Mar 15;108(11):4388-93. doi: 10.1073/pnas.1012861108. Epub 2011 Feb 28. Proc Natl Acad Sci U S A. 2011. PMID: 21368166 Free PMC article.
-
Incorporation of RG1 epitope concatemers into a self-adjuvanting Flagellin-L2 vaccine broaden durable protection against cutaneous challenge with diverse human papillomavirus genotypes.Vaccine. 2017 Sep 5;35(37):4942-4951. doi: 10.1016/j.vaccine.2017.07.086. Epub 2017 Aug 1. Vaccine. 2017. PMID: 28778613 Free PMC article.
-
A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2.PLoS One. 2011;6(8):e23310. doi: 10.1371/journal.pone.0023310. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858066 Free PMC article.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
Cited by
-
Genetic instability and anti-HPV immune response as drivers of infertility associated with HPV infection.Infect Agent Cancer. 2021 May 10;16(1):29. doi: 10.1186/s13027-021-00368-1. Infect Agent Cancer. 2021. PMID: 33971936 Free PMC article. Review.
-
Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases.Vaccines (Basel). 2020 Oct 1;8(4):568. doi: 10.3390/vaccines8040568. Vaccines (Basel). 2020. PMID: 33019516 Free PMC article. Review.
-
An update on one-dose HPV vaccine studies, immunobridging and humoral immune responses - A meeting report.Prev Med Rep. 2023 Aug 14;35:102368. doi: 10.1016/j.pmedr.2023.102368. eCollection 2023 Oct. Prev Med Rep. 2023. PMID: 37680853 Free PMC article.
-
Improvement of RG1-VLP vaccine performance in BALB/c mice by substitution of alhydrogel with the next generation polyphosphazene adjuvant PCEP.Hum Vaccin Immunother. 2021 Aug 3;17(8):2748-2761. doi: 10.1080/21645515.2021.1875763. Epub 2021 Feb 11. Hum Vaccin Immunother. 2021. PMID: 33573433 Free PMC article.
-
A Mosquito AgTRIO Monoclonal Antibody Reduces Early Plasmodium Infection of Mice.Infect Immun. 2022 Jan 25;90(1):e0035921. doi: 10.1128/IAI.00359-21. Epub 2021 Nov 1. Infect Immun. 2022. PMID: 34724388 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

