Analgecine, the extracts of Vaccinia-inoculated rabbit skin, effectively alleviates the chronic low back pain with little side effect - A randomized multi-center double-blind placebo-controlled phase 3 clinical trial
- PMID: 29736443
- PMCID: PMC5935851
- DOI: 10.1016/j.conctc.2015.11.002
Analgecine, the extracts of Vaccinia-inoculated rabbit skin, effectively alleviates the chronic low back pain with little side effect - A randomized multi-center double-blind placebo-controlled phase 3 clinical trial
Abstract
Background: Chronic low back pain affects daily activities at home and workplaces and causes a huge economic burden. Current therapeutic options are very limited and the effects of available pharmacological agents are less than satisfactory. While NSAIDs might be effective for the short term and opioids might help with urgent pain relief and improving the life quality, their long-term use is associated with significant side effects and drug misuse or abuse. To seek alternative pharmacological agents for effective treatment, we examined the therapeutic potential of the extracts of Vaccinia variola-inoculated rabbit skin (Analgecine, abbreviated as AGC) in patients with chronic low back pain due to degenerative vertebral disorders.
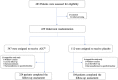
Methods: In this randomized multi-center double-blind placebo-controlled phase 3 clinical trial (Chinese Clinical Trial Registry number 2009L01498), we enrolled patients (aged 26-70 years) with chronic low back pain for at least 3 months due to degenerative spinal (vertebral) disorders from 7 medical centers in China, and randomly allocated 459 participants to receive oral AGC or placebo for 28 days to study the efficacy and safety of AGC. Randomization was performed according to a centralized randomization schedule, which was blocked by study sites and generated by an unmasked statistician independent of study conduct and data analysis. Both participants and staff at each study site were masked to treatment assignment. The primary efficacy endpoint was the change of the mean pain intensity, based on an 11-point numerical rating scale, between the baseline and the last week of treatment, with the primary efficacy analysis of intention to treat. The ratio between exposed and unexposed groups was designed to be 3:1 in order to increase the likelihood of demonstrating the AGC effect upon repeated measures.
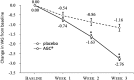
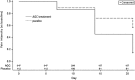
Results: 347 patients were assigned to receive AGC (4 units/tablet; 2 tablets twice a day) and 112 patients were to take placebo. Among them, 324 patients taking AGC and 112 receiving placebo completed the assessment. Patients receiving AGC reported significant pain relief at the end of week 2 and 3 compared to those taking placebo, with mean reduction of the pain scores as 1.7 vs. 0.9 at week 2 (p < 0.0001) and 2.8 vs. 1.2 at week 3 (p < 0.0001). A total of 47 AGC-treated patients reported 85 treatment emergent adverse events while 16 patients taking placebo reported 26 events, but no serious side effects were found to be related to AGC treatment.
Conclusion: Analgecine (AGC, 8 units twice daily) effectively alleviates chronic low back pain due to degenerative vertebral disorders when compared to placebo and is well tolerated by tested individuals, and can be considered as a first-line treatment for chronic low pain due to degenerative vertebral diseases.
Keywords: AGC; Analgecine; Analgesia; Chronic low back pain; Vertebral disorder.
Figures
Similar articles
-
EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial.Lancet. 2014 May 10;383(9929):1637-1647. doi: 10.1016/S0140-6736(13)62337-5. Epub 2014 Feb 5. Lancet. 2014. PMID: 24507377 Clinical Trial.
-
Fentanyl buccal tablet for the relief of breakthrough pain in opioid-tolerant adult patients with chronic neuropathic pain: a multicenter, randomized, double-blind, placebo-controlled study.Clin Ther. 2007 Apr;29(4):588-601. doi: 10.1016/j.clinthera.2007.04.007. Clin Ther. 2007. PMID: 17617282 Clinical Trial.
-
A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of the extended-release tramadol hydrochloride/acetaminophen fixed-dose combination tablet for the treatment of chronic low back pain.Clin Ther. 2013 Nov;35(11):1830-40. doi: 10.1016/j.clinthera.2013.09.017. Epub 2013 Oct 30. Clin Ther. 2013. PMID: 24183364 Clinical Trial.
-
Fentanyl buccal tablet.Drugs Today (Barc). 2008 Jan;44(1):41-54. doi: 10.1358/dot.2008.44.1.1178469. Drugs Today (Barc). 2008. PMID: 18301803 Review.
-
Methods of Treating Chronic Pain: A Systematic Review [Internet].Stockholm: Swedish Council on Health Technology Assessment (SBU); 2006 Oct. SBU Yellow Report No. 177/1+2. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2006 Oct. SBU Yellow Report No. 177/1+2. PMID: 28876750 Free Books & Documents. Review.
Cited by
-
Analgecine enhances the anti-tumor response of radiotherapy by increasing apoptosis and cell cycle arrest in non-small cell lung cancer.Oncotarget. 2017 Aug 7;8(46):80730-80740. doi: 10.18632/oncotarget.19968. eCollection 2017 Oct 6. Oncotarget. 2017. PMID: 29113340 Free PMC article.
References
-
- Waterman B.R., Belmont P.J., Jr., Schoenfeld A.J. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J. Off. J. North Am. Spine Soc. 2012;12(1):63–70. - PubMed
-
- Itz C.J., Geurts J.W., van Kleef M., Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur. J. Pain. 2013;17(1):5–15. - PubMed
-
- Chou R., Huffman L.H., American Pain S. American College of P. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann. Intern. Med. 2007;147(7):505–514. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

