Quantitative Control of GPCR Organization and Signaling by Endocytosis in Epithelial Morphogenesis
- PMID: 29731302
- PMCID: PMC6934411
- DOI: 10.1016/j.cub.2018.03.068
Quantitative Control of GPCR Organization and Signaling by Endocytosis in Epithelial Morphogenesis
Abstract
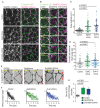
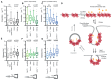
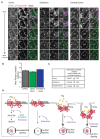
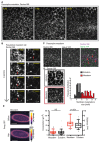
Tissue morphogenesis arises from controlled cell deformations in response to cellular contractility. During Drosophila gastrulation, apical activation of the actomyosin networks drives apical constriction in the invaginating mesoderm and cell-cell intercalation in the extending ectoderm. Myosin II (MyoII) is activated by cell-surface G protein-coupled receptors (GPCRs), such as Smog and Mist, that activate G proteins, the small GTPase Rho1, and the kinase Rok. Quantitative control over GPCR and Rho1 activation underlies differences in deformation of mesoderm and ectoderm cells. We show that GPCR Smog activity is concentrated on two different apical plasma membrane compartments, i.e., the surface and plasma membrane invaginations. Using fluorescence correlation spectroscopy, we probe the surface of the plasma membrane, and we show that Smog homo-clusters in response to its activating ligand Fog. Endocytosis of Smog is regulated by the kinase Gprk2 and β-arrestin-2 that clears active Smog from the plasma membrane. When Fog concentration is high or endocytosis is low, Smog rearranges in homo-clusters and accumulates in plasma membrane invaginations that are hubs for Rho1 activation. Lastly, we find higher Smog homo-cluster concentration and numerous apical plasma membrane invaginations in the mesoderm compared to the ectoderm, indicative of reduced endocytosis. We identify that dynamic partitioning of active Smog at the surface of the plasma membrane or plasma membrane invaginations has a direct impact on Rho1 signaling. Plasma membrane invaginations accumulate high Rho1-guanosine triphosphate (GTP) suggesting they form signaling centers. Thus, Fog concentration and Smog endocytosis form coupled regulatory processes that regulate differential Rho1 and MyoII activation in the Drosophila embryo.
Keywords: G protein-coupled receptor; G protein-coupled receptor kinase; acto-myosin contractility; endocytosis; epithelial morphogenesis; fluorescence correlation spectroscopy; gastrulation; β-arrestin.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Distinct RhoGEFs Activate Apical and Junctional Contractility under Control of G Proteins during Epithelial Morphogenesis.Curr Biol. 2019 Oct 21;29(20):3370-3385.e7. doi: 10.1016/j.cub.2019.08.017. Epub 2019 Sep 12. Curr Biol. 2019. PMID: 31522942 Free PMC article.
-
Multifunctional role of GPCR signaling in epithelial tube formation.Development. 2022 Aug 1;149(15):dev200519. doi: 10.1242/dev.200519. Epub 2022 Aug 11. Development. 2022. PMID: 35876688
-
Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis.Nat Cell Biol. 2016 Mar;18(3):261-70. doi: 10.1038/ncb3302. Epub 2016 Jan 18. Nat Cell Biol. 2016. PMID: 26780298
-
The cellular and molecular mechanisms that establish the mechanics of Drosophila gastrulation.Curr Top Dev Biol. 2020;136:141-165. doi: 10.1016/bs.ctdb.2019.08.003. Epub 2019 Sep 13. Curr Top Dev Biol. 2020. PMID: 31959286 Review.
-
Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases.Life Sci. 2003 Dec 5;74(2-3):225-35. doi: 10.1016/j.lfs.2003.09.009. Life Sci. 2003. PMID: 14607250 Review.
Cited by
-
The pulse of morphogenesis: actomyosin dynamics and regulation in epithelia.Development. 2020 Sep 2;147(17):dev186502. doi: 10.1242/dev.186502. Development. 2020. PMID: 32878903 Free PMC article. Review.
-
The Drosophila melanogaster Rab GAP RN-tre cross-talks with the Rho1 signaling pathway to regulate nonmuscle myosin II localization and function.Mol Biol Cell. 2020 Oct 1;31(21):2379-2397. doi: 10.1091/mbc.E20-03-0181. Epub 2020 Aug 20. Mol Biol Cell. 2020. PMID: 32816624 Free PMC article.
-
Fog signaling has diverse roles in epithelial morphogenesis in insects.Elife. 2019 Oct 1;8:e47346. doi: 10.7554/eLife.47346. Elife. 2019. PMID: 31573513 Free PMC article.
-
Orchestrating morphogenesis: building the body plan by cell shape changes and movements.Development. 2020 Sep 11;147(17):dev191049. doi: 10.1242/dev.191049. Development. 2020. PMID: 32917667 Free PMC article. Review.
-
Combinatorial patterns of graded RhoA activation and uniform F-actin depletion promote tissue curvature.Development. 2021 Jun 1;148(11):dev199232. doi: 10.1242/dev.199232. Epub 2021 Jun 14. Development. 2021. PMID: 34124762 Free PMC article.
References
-
- Heisenberg C-P, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. - PubMed
-
- Munjal A, Lecuit T. Actomyosin networks and tissue morphogenesis. Development. 2014;141:1789–1793. - PubMed
-
- Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. - PubMed
-
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

