The impact of phosphatases on proliferative and survival signaling in cancer
- PMID: 29725697
- PMCID: PMC6023766
- DOI: 10.1007/s00018-018-2826-8
The impact of phosphatases on proliferative and survival signaling in cancer
Abstract
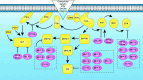
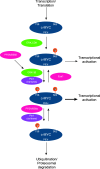
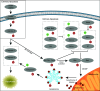
The dynamic and stringent coordination of kinase and phosphatase activity controls a myriad of physiologic processes. Aberrations that disrupt the balance of this interplay represent the basis of numerous diseases. For a variety of reasons, early work in this area portrayed kinases as the dominant actors in these signaling events with phosphatases playing a secondary role. In oncology, these efforts led to breakthroughs that have dramatically altered the course of certain diseases and directed vast resources toward the development of additional kinase-targeted therapies. Yet, more recent scientific efforts have demonstrated a prominent and sometimes driving role for phosphatases across numerous malignancies. This maturation of the phosphatase field has brought with it the promise of further therapeutic advances in the field of oncology. In this review, we discuss the role of phosphatases in the regulation of cellular proliferation and survival signaling using the examples of the MAPK and PI3K/AKT pathways, c-Myc and the apoptosis machinery. Emphasis is placed on instances where these signaling networks are perturbed by dysregulation of specific phosphatases to favor growth and persistence of human cancer.
Keywords: Apoptosis; MAPK; PI3K; PP2A; Phosphorylation; c-Myc.
Figures
Similar articles
-
A Crosstalk Between Dual-Specific Phosphatases and Dual-Specific Protein Kinases Can Be A Potential Therapeutic Target for Anti-cancer Therapy.Adv Exp Med Biol. 2021;1275:357-382. doi: 10.1007/978-3-030-49844-3_14. Adv Exp Med Biol. 2021. PMID: 33539023
-
PI(4,5)P2 5-phosphatase A regulates PI3K/Akt signalling and has a tumour suppressive role in human melanoma.Nat Commun. 2013;4:1508. doi: 10.1038/ncomms2489. Nat Commun. 2013. PMID: 23443536 Free PMC article.
-
Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases.Biosci Rep. 2017 Feb 10;37(1):BSR20160432. doi: 10.1042/BSR20160432. Print 2017 Feb 28. Biosci Rep. 2017. PMID: 28082369 Free PMC article. Review.
-
Restoration of SHIP activity in a human leukemia cell line downregulates constitutively activated phosphatidylinositol 3-kinase/Akt/GSK-3beta signaling and leads to an increased transit time through the G1 phase of the cell cycle.Leukemia. 2004 Nov;18(11):1839-49. doi: 10.1038/sj.leu.2403529. Leukemia. 2004. PMID: 15457186
-
Mechanisms of ROS modulated cell survival during carcinogenesis.Cancer Lett. 2008 Jul 18;266(1):30-6. doi: 10.1016/j.canlet.2008.02.029. Epub 2008 Mar 26. Cancer Lett. 2008. PMID: 18372105 Review.
Cited by
-
IGF‑IR promotes clonal cell proliferation in myelodysplastic syndromes via inhibition of the MAPK pathway.Oncol Rep. 2020 Sep;44(3):1094-1104. doi: 10.3892/or.2020.7652. Epub 2020 Jun 19. Oncol Rep. 2020. PMID: 32583001 Free PMC article.
-
Review of PP2A Tumor Biology and Antitumor Effects of PP2A Inhibitor LB100 in the Nervous System.Cancers (Basel). 2021 Jun 21;13(12):3087. doi: 10.3390/cancers13123087. Cancers (Basel). 2021. PMID: 34205611 Free PMC article. Review.
-
Loss of PR55α promotes proliferation and metastasis by activating MAPK/AKT signaling in hepatocellular carcinoma.Cancer Cell Int. 2021 Feb 15;21(1):107. doi: 10.1186/s12935-021-01796-0. Cancer Cell Int. 2021. PMID: 33588847 Free PMC article.
-
Targeting PP2A in cancer: Combination therapies.Biochim Biophys Acta Mol Cell Res. 2019 Jan;1866(1):51-63. doi: 10.1016/j.bbamcr.2018.08.020. Epub 2018 Sep 1. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30401535 Free PMC article. Review.
-
Targeting AKT/PKB to improve treatment outcomes for solid tumors.Mutat Res. 2020 Jan-Apr;819-820:111690. doi: 10.1016/j.mrfmmm.2020.111690. Epub 2020 Feb 20. Mutat Res. 2020. PMID: 32120136 Free PMC article. Review.
References
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. - DOI - PubMed
-
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–362. doi: 10.1038/ng0497-356. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

